- within Energy and Natural Resources, Tax and International Law topic(s)
- with readers working within the Media & Information industries
Introduction
Regulations on chemical substances play a pivotal role in our daily lives for the protection of human health and the environment as well as for the assessment and mitigation of hazardous effects and risks of chemicals. For this reason, the Regulation on the Registration, Evaluation, Authorization, and Restriction of Chemicals1 ("REACH") governing the manufacture, use, and import of chemical substances in the European Union ("EU") was adopted and the European Chemicals Agency ("ECHA") established by the Regulation is the responsible authority to ensure the implementation of the REACH. Following the REACH Regulation, many similar regulations concerning chemicals entered into force worldwide, and the KKDIK Regulation (Kimyasalların Kaydı, Değerlendirilmesi, İzni ve Kısıtlanması Hakkında Yönetmelik)2, also known as Turkish REACH, was adopted in Turkey. The KKDIK Regulation was directly modelled after the REACH for the harmonization of the chemical safety regulations in Turkey with the EU and aligns closely with its provisions and requirements.
Based on the principle of "no data, no market", chemical substances within the scope of the REACH and KKDIK must be registered by providing the required information by the regulation through the chemicals registration system, REACH-IT of ECHA in the EU and KKS3(Kimyasal Kayıt Sistemi) of the Turkish Ministry of Environment, Urbanization and Climate Change ("Ministry") in Turkey. No permission or restriction has been granted by the Ministry to use or not use the information in the registration dossiers submitted under the REACH Regulation in the EU. There is no agreement between the Ministry and ECHA on this matter, therefore, a registration of chemicals under the REACH will not eliminate any registration obligation under the KKDIK Regulation.
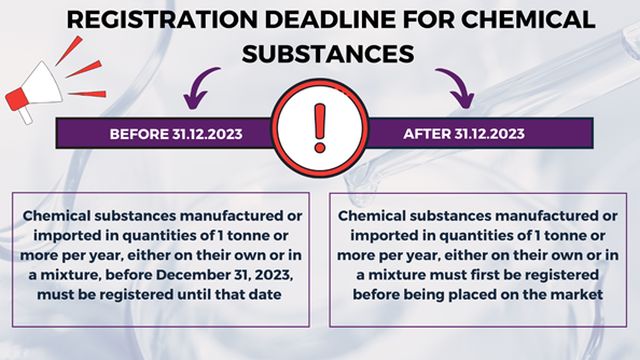
The obligations and duties set forth by the KKDIK Regulation gain more importance for companies operating in the chemicals industry in Turkey considering the registration deadline for chemicals that will expire on December 31, 2023. According to the KKDIK Regulation, substances manufactured or imported in quantities of 1 tonne or more per year, either on their own or in a mixture, before December 31, 2023, must be registered until that date. After December 31, 2023, substances must first be registered before being placed on the market. Therefore, as of January 1, 20124, the chemical substances that are not registered to the KKS will not be allowed to be manufactured or imported.
Considering the critical importance of the registration of chemicals in accordance with the KKDIK Regulation, the registrants of the same substance are obliged to file a joint registration as per the principle of "one substance, one registration". At this point, two main agreements come to the fore as requirements, which are the "Data Sharing Agreement" and the "Joint Registration Agreement". The purpose of these agreements is to share data among the registrants of the same substance to minimize the use of animals and avoid unnecessary testing for the same chemicals. This article will mainly focus on the Data Sharing Agreement as its content may be confused with other similar agreements (e.g. Agreement on Letter of Access).
Legal Background and the Scope
The REACH which entered into force on June 1, 2007, in the EU, and the KKDIK Regulation on December 23, 2017, in Turkey aim to address and enhance the protection of human health and the environment while fostering innovation and competitiveness of the chemical industry as it is one of the key sectors. The REACH and KKDIK regulations encompass the registration and evaluation of chemicals, authorization processes for hazardous substances, and the restriction of certain substances in articles. Therefore, the hazards and risks of chemical substances also need to be assessed before being placed on the market. The chemicals within the scope of the REACH and KKDIK regulations in quantities of 1 tonne or more per year must be registered in the EU and Turkey regardless of whether they are used on their own, in a mixture, or an article.
Since the main principle for the registration of chemical substances is "one substance, one registration", manufacturing and importing companies are required to manage the joint registration and data sharing of the same substance. To ensure that companies fulfill their duties and obligations, the Guidance on Data Sharing and Registration4 and Implementing Regulation 2016/9 on joint submission of data and data sharing5 were issued in the EU, and the Data Sharing Guidance ("Guidance")6 and Joint Submission of Data and Data Sharing Rules Under KKDIK ("Rules") 7 rules updated by the Ministry in Turkey on April 25, 20238, are published as the translations of the corresponding guidelines of the REACH. Accordingly, the Ministry has recently announced that companies need to assess and determine whether their chemical substance, mixtures, or materials are subject to registration under KKDIK and warns the companies to not apply to the Ministry for written approval.9 In this regard, the Guidance and the Rules published by the Ministry become crucial for the companies to self-assess the chemicals to be manufactured, used, or imported in Turkey to ensure that they fulfill their obligations under the KKDIK Regulation.
The alignment of requirements and procedures promotes consistency, streamlines regulatory processes, and reduces compliance burdens for businesses operating in both Turkey and the EU. On the other hand, it should be noted that according to Turkish legislation, manufacturers located outside Turkey do not have direct obligations under the KKDIK Regulation. The importer resident in Turkey must fulfill the KKDIK Regulation obligations.
Data Sharing Agreements
As mentioned above, under the KKDIK Regulation, in case there is more than one registrant for the registration of the same substance, then they are required to submit a joint registration and share their data and related costs with other registrants in a fair, transparent, and non-discriminatory way.
The purpose of a data-sharing agreement is to reduce registration costs for the same substance, increase the efficiency of the registration system of chemicals, and avoid any unnecessary testing, especially on vertebrate animals.
The language of the agreement and the governing law for the dispute settlement mechanism could be agreed by the parties within the context of the freedom of contract and choice of law.
The Rules together with the Guidance set forth the requirements in the management of Data Sharing Agreements for the registration of the same substance under the KKDIK Regulation as well as the duties and obligations of the parties to the agreement. Under the Turkish Code of Obligations, Data Sharing Agreements are multilateral innominate contracts that impose debt on both parties. The Ministry does not intervene in the content of this Agreement and the parties are required to prepare the agreements within the scope of freedom of contract.
Under the Rules, parties to a Data Sharing Agreement will consist of the owner of the data and the grantee who may either be an existing or potential registrant. The Rules also stipulate the sections to be included in the Data Sharing Agreement related to cost calculation and reimbursement mechanisms.
To reach an agreement on cost sharing, parties need to agree on
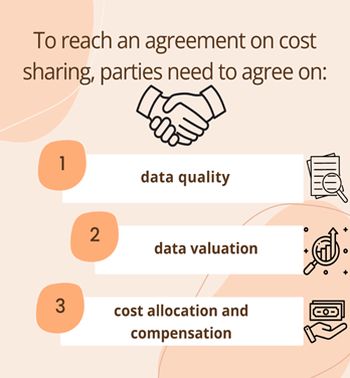
- the data quality
- data valuation
- and cost allocation and compensation regarding how the agreed value will be shared among the parties.
These mandatory elements to be included in the Data Sharing Agreement could only be waived by the unanimous agreement of the co-registrants including the potential future ones. However, it should be noted that a potential registrant will not be bound by the waiver in an existing agreement unless an agreement is reached.
Any form of cooperation could be organized by the potential registrants regarding the data sharing and joint registration obligations. That said, although a formal agreement in writing or a consortium is not legally required, KKDIK guidance on data sharing advises that parties agree in writing (even by e-mail) on the main rules of data sharing, on the ownership of the studies jointly developed and on the sharing of costs. In practice, various bilateral agreements could be established within a consortium, between different members of the registration, or with external data holders. At this point, it should also be noted that parties need to ensure that data sharing and information exchange is limited to what is required under KKDIK in order to avoid any risk of competition law infringement and the exchange should not go beyond the KKDIK requirements.
The Data Sharing Agreement may be confused with other agreements to which the content is similar, in particular with an Agreement on Letter of Access. According to the Guidance, an agreement on a Letter of Access ("LoA") or a license to use grants the right to refer to the full study report for registration purposes. According to the Guidance, a LoA would be used when a registrant does not own a study report for their registration requirement and agrees with the owner on the conditions of using the study report to fulfill their registration obligation under KKDIK. Therefore, a LoA does not grant ownership of the data. The agreement may take the form of a license to use when the study report that is requested to be referred is subject to copyright or confidential business information.
Conclusion
In conclusion, compliance with the KKDIK Regulation is highly important to ensure a high level of protection of human health and the environment, to encourage alternative methods of assessing the hazards of substances, to minimize the use of animals, and to increase competition and innovation. In that sense, fulfilling the obligations required by the KKDIK Regulation as regards data sharing and joint registration should be on the agenda of the companies operating in the chemicals industry considering the approaching deadline for the registration of chemicals. As stated above, compliance with competition law rules should be ensured by the companies while carrying out their obligations under KKDIK including data sharing and information exchange. It should also be noted that data sharing and joint registration agreements that are compliant with the REACH Regulation would also facilitate the registration process in Turkey for the relevant chemicals and companies could contact our firm for legal assistance to ensure full compliance with the KKDIK Regulation.
Footnotes
1. https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:32006R1907R(01)
2. https://www.mevzuat.gov.tr/mevzuat?MevzuatNo=23694&MevzuatTur=7&MevzuatTertip=5
3. https://kimyasallar.csb.gov.tr/kimyasal-kayit-sistemi-kks/321
5. https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A32016R0009
6. https://webdosya.csb.gov.tr/db/cygm/icerikler/ver--paylasim-rehber--25.04.2023-20230425130648.pdf
7. https://webdosya.csb.gov.tr/db/cygm/icerikler/ver--paylasimi-kurallari-25.04.2023 20230425130714.pdf
8. https://kimyasallar.csb.gov.tr/veri-paylasimi-rehberi-guncellendi/366
9. https://kimyasallar.csb.gov.tr/kkdik-onemli-duyuru/368
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.


