- within Environment, Wealth Management and Real Estate and Construction topic(s)
On March 19, 2025, China's National Medical Products Administration (NMPA) released the Draft Measures for the Protection of Drug Trial Data (the "Draft Measures"), along with its accompanying implementation guidelines, the Working Procedures for Drug Trial Data Protection, for public consultation.
Back in 2002, China first introduced the concept of a six-year data protection period in the Implementing Regulations to the Drug Administration Law, though detailed rules were lacking. In 2018, the NMPA proposed the Draft Implementing Measures for Drug Trial Data Protection (Interim) (the "2018 Draft"), which outlined a more comprehensive classification system for data protection — including provisions such as a 12-year protection period for innovative biologics. However, due to unresolved controversies, the 2018 Draft never came into effect.
The Draft Measures this year signal a calibrated effort to finally launch a data protection regime in China and to align with international standards. Overall, the Draft Measures seek to incentivize R&D investment without unduly delaying generic market entry. Set out below are the highlights of the Draft Measures.
Categories of protected drugs expanded
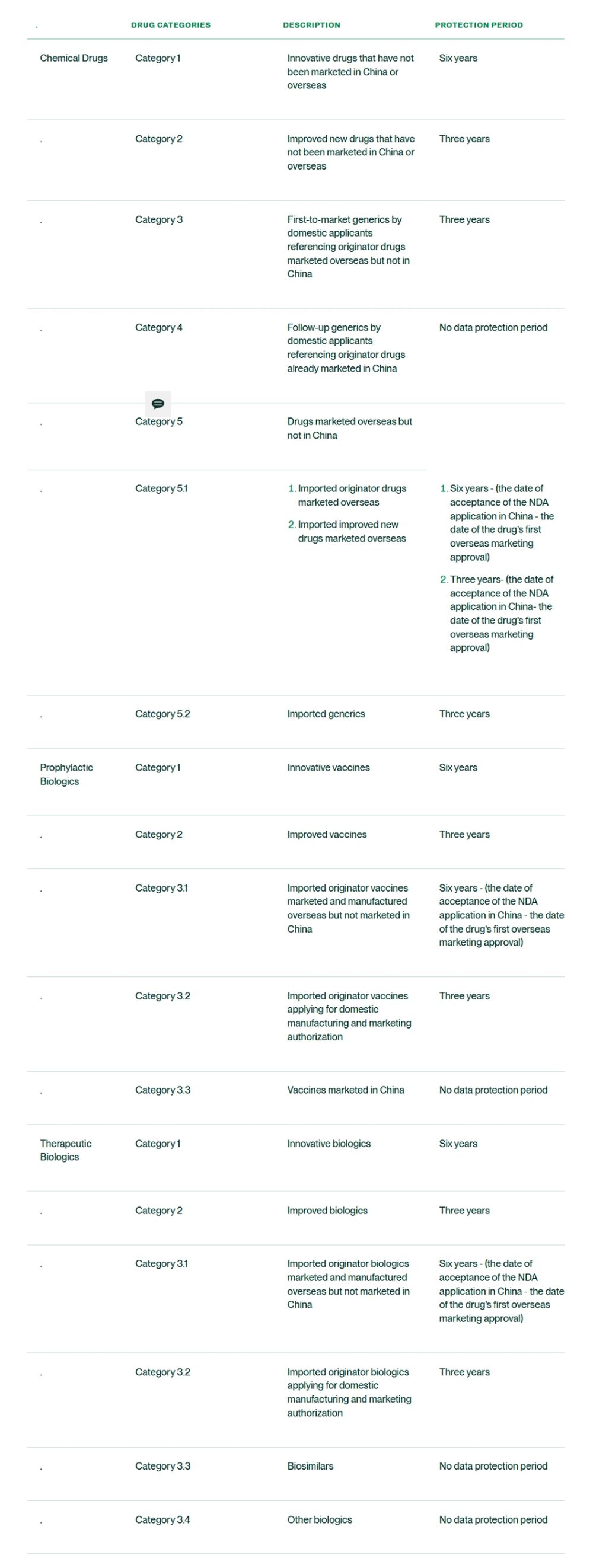
Different from 2018 Draft, the Draft Measures are silent on the data protection period for orphan drugs and pediatric drugs. However, they significantly expand the scope of data protection to encompass first-to-market generics and certain prophylactic biologics.
Protection periods of innovative therapeutic biologics aligned with chemical drugs
Compared to the 12-year protection for innovative therapeutic biologics under the 2018 Draft, the Draft Measures take a more moderate approach by aligning the protection periods for innovative therapeutic biologics with that for innovative chemical drugs. Both categories are now granted a six-year data protection period.
Mechanism for accelerating MNCs' product launch in China
The 2025 Draft provides for a mechanism designed to encourage pharmaceutical companies to accelerate the product launch and market entry of imported new drugs in China. Specifically, for drugs that have been approved overseas but are not yet marketed in China, the time difference between the date of NDA acceptance in China and the date of the first overseas marketing approval will be deducted from the full data protection period for innovative and improved products.
For specific categories and data protection periods, please refer to the table below.
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.