- within Intellectual Property topic(s)
- in Asia
Pharmaceuticals and agricultural drugs cannot be marketed for sale in Taiwan before a regulatory approval is obtained. Thus, in order to compensate for the delay resulting from clinical tests or obtaining regulatory approval, Taiwan's Patent Act has embraced a patent term extension system. In accordance with Article 53 of the Patent Act, if the practice of an invention patent relating to pharmaceutical or pesticide composition or the manufacturing method therefor is subject to regulatory approval and if the approval is obtained after publication of the grant of the patent, the patentee may apply for a patent term extension based upon the first certificate of regulatory approval.
In general, only one single extension is obtainable, and a certificate of regulatory approval can be based upon to apply for an extension just for once. Moreover, the duration of a patent extension is not allowed to go beyond the duration during which the patent cannot be practiced due to the need to obtain regulatory approval. While an extension application must be filed within three months from the date of issuance of the first certificate of regulatory approval, no application is available within six months prior to the expiry date of the patent term.
The patent extension system has been in operation in Taiwan for more than 25 years. It is therefore an appropriate time to take stock of the situation as it is so as to find a way forward. Below is the result of a search and analysis performed based on the data available in the Patent Gazette prior to February 2020, and the issuance date of the first certificates of regulatory approval after January 1, 2010. The search did not cover the applications that have not yet been indexed by Taiwan's IP Office (TIPO), since there is a time lag between the date an application is filed and the date it is entered into the database after the formalities are fulfilled. Under Taiwan's Patent Act, there is a grace period of 6 months for submission of the requisite documents on a late basis.
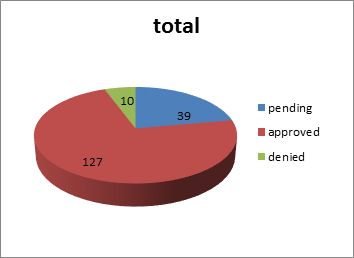
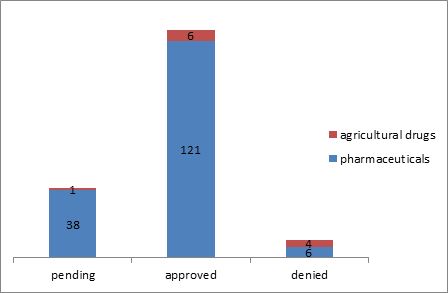
The search located a total of 176 extension applications. It was found that 165 or approximately 93.75 percent of the 176 applications related to pharmaceuticals (38 pending, 121 approved and 6 denied), while 11 or 6.25 percent related to agricultural drugs (1 pending, 6 approved and 4 denied.)
Please see the chart below:
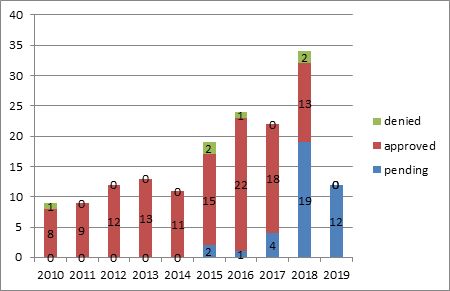
Of the 6 approved applications relating to agricultural drugs, two requested an extension of 5 years and the other 4 requested an extension of 2-4 years. One Of the 2 applications was granted an extension of 5 years as requested and the other was granted an extension of a period of several months less than 5 full years. In addition, 3 of the 4 denied applications were those filed with a request for an extension of 5 years, and 1 of them was denied due to the patentee's failure to pay the annuity to maintain the validity of the patent. There has been an increase in the number of applications relating to pharmaceuticals of which the certificates of regulatory approval were obtained after 2015. Although the total number of applications filed on the basis of the certificates of regulatory approval issued in 2019 slightly decreased, yet there were excluded from the search quite a number of applications not docketed into the database due to the time lag mentioned above. In addition, most of the pending applications were those filed on the basis of the certificates of regulatory approval issued in 2018 or 2019.
Please refer to the chart below:
Of the 165 applications relating to pharmaceuticals, 64 were filed requesting an extension of 5 years. As for the number of applications requesting an extension of less than five years, please refer to the chart below:
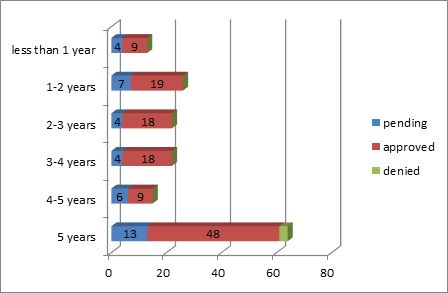
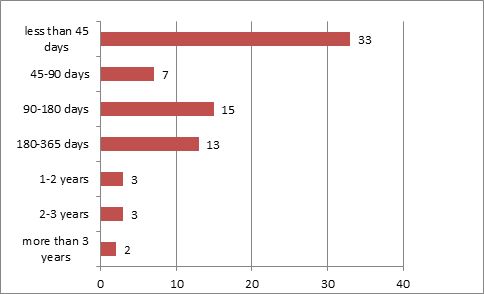
45 or 37.19 percent of the 165 applications were granted an extension of time as requested. 36 or 80 percent of the 45 applications were granted a maximum of five years as requested. For nearly 30 percent of the applications, there was only a 45-day interval between the requested and approved durations.
Please refer to the chart below:
Of the 6 denied applications, 3 new applications were further filed at a later time. While one of them was approved in April 2020, the other two were still pending.
It can be learnt from the above data that, compared with agricultural drugs, the approval rate for pharmaceuticals is much higher, and two-thirds of the approved applications relating to pharmaceuticals obtained a time extension with only a 45-day interval between the requested and approved durations. Therefore, it will be of great help in ensuring the right or best interest of the patentee if a patent term extension application is filed at the soonest after obtaining the first certificate of regulatory approval. One other matter which merits the patentee's attention is that annuities must always be paid in a timely manner.
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.

