- within Compliance topic(s)
- in Asia
- with readers working within the Pharmaceuticals & BioTech industries
Since patent linkage came into effect on 1 June 2021 (see, Luo JH et al, Why brand-name pharmaceuticals should look to China's patent linkage system), more than 100 patent linkage cases have been filed and dozens of decisions have been issued by the Chinese National Intellectual Property Administration (CNIPA) and courts. Recently, CNIPA and National Healthcare Security Administration (NHSA) jointly issued the Opinions on Strengthening the Protection of Intellectual Property Rights in the Field of Medical Centralised Procurement (the Opinions) on 30 December 2022. In this article, we provide a summary of the new developments in patent linkage practices in China and explore what the first decisions tell us about the direction of travel.
A quick review of the patent linkage
The marketing authorisation holders (MAHs) of originator drugs can list their patents covering the originator drugs in a patent linkage platform. Generic applicants (ANDA filers) must submit one of the following four types of certifications for each patent listed with the ANDA and inform the MAH:
- Type 1: no patent information related to the reference-listed drug (brand-name drug) on the Registration Platform;
- Type 2: the patent has been terminated or declared invalid, or the generic drug applicant has obtained a patent licence from the patentee;
- Type 3: the generic drug applicant promises that the generic drug will not be marketed before the expiration of the listed patent; and
- Type 4: the patent shall be declared invalid (Type 4.1), or the generic drug is not covered by the protection scope of the patent (Type 4.2).
For Type 4 certifications, the patentee or MAH can take legal action before the Beijing IP Court (civil action route) or request an administrative adjudication with CNIPA (administrative adjudication route) within 45 days. A nine-month waiting period is thereby triggered, during which the ANDA cannot be approved. After the waiting period, the National Medical Products Administration (NMPA) will approve or postpone the approval of the generic according to the decision of the litigation.
Summary of the related cases of the patent linkage
There are 1,180 drugs (including different strengths) for which patents have been listed as shown below (according to data from Zhichanbao database in 26 May 2023).
| Drug type | Drugs with listed patents |
| Chemical drugs | 722 |
| Biological products | 118 |
| Traditional Chinese medicine | 340 |
| Total | 1,180 |
There are 4,886 patent certifications published in the patent
linkage platform as shown below (data from Zhichanbao database in
26 May 2023).
| Drug type | Drugs with listed patents |
| Chemical drugs | 4,827 |
| Biological products | 58 |
| Traditional Chinese medicine | 1 |
| Total | 1,180 |

As seen from the above data, Type 1 (no patents) comprises a majority of the patent certifications (81%), while Type 4 (challenging patents) including Type 4.1 and 4.2 accounts for only 5%. It appears that there has been a shift in the trend of generic companies' preferences regarding patent linkage. Initially, when the patent linkage policy was implemented, there was a tendency for these companies to opt for Type 4.1 certifications (invalid patent) rather than Type 4.2 certifications (no coverage). However, in recent times, there has been an increase in the number of Type 4.2 certifications being filed.
Statistic of the cases of the administrative adjudication:
According to CNIPA, from 5 July 2021 to 30 April 2023, the CNIPA received 127 cases of administrative adjudication as follows.
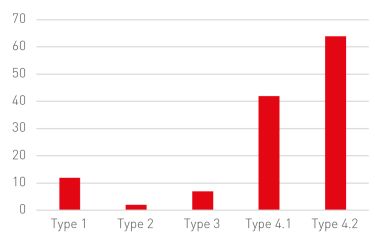
These administrative adjudication cases involved 25 brand-name companies and 43 generic companies, 30 drugs and 47 patents, with 30 patents being challenged during the examination of the cases.
The result/decision of the administrative adjudication normally includes that the:
- generic drug falls under the scope of the disputed patent;
- generic drug does not fall under the scope of the disputed patent;
- patentee or MAH withdraws the request of administrative adjudication on his or her own initiative and, therefore, the examination of the case terminates; and
- request of administrative adjudication is rejected by the CNIPA due to the disputed patent being fully invalidated or, rarely, the disputed patents being not eligible for listing on the platform (eg, patents for crystal forms) (see the table below (from our own statistics based on CNIPA data in January 2023)).
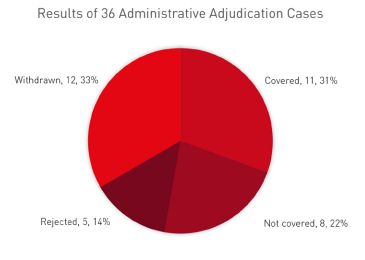
As seen from this data, the cases of generic drugs covered by the patents are higher than those not covered (31% versus 22%). Interestingly, in nearly 33% of cases the patentee or MAH voluntarily withdrew the request for administrative adjudication. The reasons could be that the patentee or MAH found the generic drug not covered by the patent after reviewing the formulation of the generic drug or decided to proceed with a parallel civil action instead of the administrative case.
Common reasons as to why CNIPA rejects requests are the patent being declared invalid in whole during the invalidation proceedings, the patent certification not being Type 4 or the patent not being listable.
Considering that the civil action route is difficult to initiate, and much slower to get a useful decision from, cases of patent linkage civil action are much less freuent than that of administrative adjudication. Recently, the judgement of the Supreme Court on the first patent linkage civil action (Chugai pharmaceutical v Wenzhou Haihe pharmaceutical, (2022) ZUI GAO FA ZHI MIN ZHONG No. 905) was made on 5 August 2022. The Supreme Court favours the generic Haihe's opinion that the generic drug was not covered by the granted claims and, therefore, does not fall into the scope of the disputed patent.
Summary of the substantial issues in patent linkage
Here are some conclusions that were drawn from recent patent linkage litigation cases (including both administrative and civil actions).
- A patent for crystal forms or the use of crystal forms is not eligible for listing. Whether a patent belongs to the patent for crystal forms depends on the subject matter and the defined features of a claim.
- Whether or not the patent listing information is accurate and authentic shall be resolved by judging whether the patent covers the technical solution of the originator drug approved for marketing. It is only required that the technical solution of the originator drug falls into the scope of the patent listed on the platform, and the 'inventive point' of the patent is not required to be expressly recited in the new drug application materials (see (2021) GUO ZHI YAO CAI No. 0023).
- The 'all element' rule, doctrine of equivalents, prosecution history estoppel and disclosure-dedication doctrine all apply to patent linkage litigation cases. In cases where at least one technical feature of the generic drug is not identical or equivalent to those in the claim of the patent, the generic drug does not fall into the scope of the patent. Where a technical solution is only described in the description, rather than in the claims, CNIPA does not support the patentee incorporating the technical solution into the scope of claims again on the ground of the doctrine of equivalents (see (2021) GUO ZHI YAO CAI No. 0021).
- The generic company has the burden to disclose the technical solutions of the generic drug. In cases where the generic applicant fails to submit evidence in support of its allegation that the generic drug does not fall into the scope of the patent, the generic applicant has to bear unfavourable consequences due to legal burden of proof (see (2022) GUO ZHI YAO CAI No. 0010).
- The indications in the package insert are a clear delimitation as to the use of a pharmaceutical for the prevention, treatment, diagnosis and auxiliary treatment of a disease or symptom. Whether or not the technical solution of a generic drug falls into the use of the disputed patent depends on the indications clearly set forth in the ANDA materials during the examination of administrative adjudication (see (2021) GUO ZHI YAO CAI No. 0012).
- As far as the product claim defined by a preparation method is concerned, the scope of the patent is limited by the features of the preparation method. The technical solution of a generic drug does not fall into the scope of the patent if the preparation method of the generic drug is not identical or equivalent to the preparation method of the patent (see (2022) GUO ZHI YAO CAI No. 0016).
The latest Opinions issued by CNIPA and the NHSA
CNIPA and the NHSA issued the Opinions on Strengthening the Protection of Intellectual Property Rights in the Field of Medical Centralised Procurement on 30 December 2022, which are favourable for patentees to enforce their patents in the field of medical centralised procurement through either normal patent litigation or patent linkage litigation.
According to the Opinions, the patentee is able to raise an objection to a product for patent infringement before the medical centralised procurement agency, which may request the local IP administration to issue an opinion of consultation or infringement determination and shall decide whether to allow the product to participate in the centralised procurement with reference to the opinion.
The medical centralised procurement agency needs to check the qualification of the product based on the information published by relevant administrations and withdraw the listing of the product in the procurement platform in a timely manner once a clear infringement is found.
It is likely that Type 3 patent certifications in patent linkage are clearly infringing the patent and will not be allowed to be listed in the platform as Type 3 patent certification promises that the generic drug will not be marketed until the expiration of patents.
Where a patent infringement dispute arises in the centralised procurement, the patentee is able to request an IP administration to deal with the dispute or take legal action before the court. The medical centralised procurement agency shall take measures to stop infringement, such as prohibiting listing, withdrawing the listing and disqualifying the selected product, according to the administrative ruling of the IP administration or the effective judgment of the court.
Takeaways
Patent linkage litigation is fast, efficient and effective, and it has some important advantages over normal patent litigation. First, patent linkage litigation can usually be concluded in a very fast manner. CNIPA normally issues decisions in nine months, and CNIPA's decisions can be used to stop the approval of generic drugs in the NMPA. Second, in patent linkage litigation, generic companies have the burden to disclose the technical solutions of the generic drug and, where the generic companies fail to do so, they have to bear unfavourable consequences. Therefore, patent linkage litigation is very efficient for patentees, as they are relieved of the burden to prove that the generic product infringes the patent, which can be very burdensome and time-consuming. Last, patent linkage litigation is very effective as the NMPA will not approve the generic product until expiration of the patent if a CNIPA decision confirms the coverage of a generic product by the patent. For a Type 3 patent certification, although the generic product will be approved, the relevant IP enforcement rules (eg, the Opinions) will likely ensure that the generic company will not be able to sell the generic product until the patent expires.
Therefore, it is advisable for patentees and MAHs to take full advantage of the patent linkage in China.
The deadline to list and update patents is 30 days from drug approval or information change. As patent listing is the first step to initiate patent linkage, and patents and claims that are not listed on the platform are not available for patent linkage in China, it is important to list all eligible patents and claims as soon as possible after the drug is approved and the patents are issued.
To take full advantage of patent listing and other pharmaceutical IP protections in China, it is suggested that when developing new drugs and applying for new drug marketing authorisations, brand-name pharmaceutical companies should conduct simultaneous global research and development, and almost simultaneously file new drug marketing applications in China and in other countries. In particular, to utilise the patent linkage, all drugs, dosage forms and strengths should be approved in China as soon as possible after they are approved in other countries. Otherwise, any drugs, dosage forms or strengths that are approved in other countries but not approved in China will not qualify for patent listing, resulting in no patent linkage being available to those particular drugs, dosage forms and strengths.
Although generic companies are supposed to inform the MAHs of patent certifications, in practice, some generic companies do not do so and generic companies' failure to inform lacks remedy for MAHs. Therefore, it is very important for MAHs to monitor patent certifications published in the patent linkage platform. Furthermore, if patent certifications are not correct, MAHs should request the NMPA to correct them, because only Type 4 patent certifications can be sued.
It is clear now that the administrative adjudication route is easier to initiate and quicker to get a useful decision, but is limited to Type 4 patent certification and has no preliminary injunction, while the civil action route is difficult to initiate and slower to get a useful decision but has preliminary injunction and may accept disputes on Types 1 and 2 patent certifications in addition to Type 4 certifications. Dual filing of two routes is possible when the request for administrative adjudication is filed prior to the filing of a civil lawsuit by the 45-day time limit. Most originators chose the administrative adjudication, and a few chose the civil action or the dual filing.
It is common for more than one generic company to file an ANDA and a Type 4 patent certification for the same originator drug and for more than one generic company to challenges the validity of a patent. As a result, originators may face more than one patent linkage litigation case and more than one patent invalidation case at the same time, and thus could have very tight timelines. Therefore, it is suggested that originators have adequate preparedness (eg, on which of the MAHs, patentees or licensees are to take action, who has the right to sign power of attorney and proof to show the right to sign, how to notarise and legalise the formality documents, how to prove coverage of originator drugs by patents, how to construe claims of the patents and how to respond to validity challenges).
Originally published by IAM.
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.


