- within Media, Telecoms, IT and Entertainment topic(s)
- in United States
In light of growing concerns regarding electronic cigarettes, vapes, or, technically, Electronic Nicotine Delivery Systems (ENDS), Similar Non-Nicotine Systems (SNNS), Heated Tobacco Products (HTPs), and Oral Nicotine Products (ONPs), regulatory intervention has finally occurred, equating these products with cigarettes, tobacco products, and their derivatives.
That is to say, as of May 9, 2025, electronic cigarettes, vapes,
and similar devices shall not:
a) be marketed to minors or be made especially appealing to
them;
b) suggest that smoking contributes to athletic or sporting
success, popularity, professional success, or sexual success;
c) contain false or misleading advertising, including the use of
terms such as "mild," "light,"
"low-tar," "low-nicotine," "low-carbon
monoxide," or similar descriptors.
The promotion of these products is prohibited through the use of billboards, banners, murals, posters, placards, or similar media, whether fixed or mobile, as well as through radio, television, cinema, printed media such as bulletins, newspapers, magazines, or any form of mass distribution.
Finally, as with cigarettes, tobacco products, and their derivatives, Electronic Nicotine Delivery Systems (ENDS), Similar Non-Nicotine Systems (SNNS), Heated Tobacco Products (HTPs), and Oral Nicotine Products (ONPs) must include health warnings and pictograms issued by the Ministry of Health, some of which for the current year are as follows:
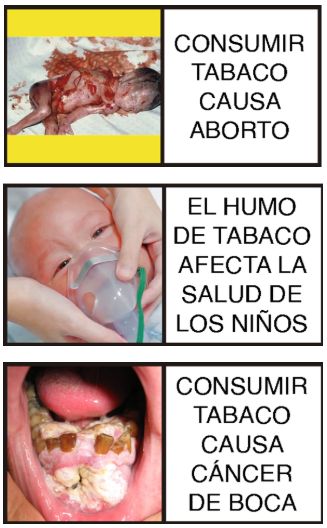
Following the regulatory equalization of Electronic Nicotine Delivery Systems (ENDS), Similar Non-Nicotine Systems (SNNS), Heated Tobacco Products (HTPs), and Oral Nicotine Products (ONPs) with cigarettes, tobacco products, and their derivatives under Law 2354 of 2024, various implementing regulations were issued.
The first was issued by the Ministry of Health and Social Protection through Resolution No. 624 of April 8, 2025, which adopted the Manual for the Signage of 100% Smoke-Free Environments and Aerosols Emitted by Substitutes and Imitators. This Manual applies to indoor areas of workplaces and/or public places, such as: bars, restaurants, shopping centers, stores, fairs, festivals, parks, stadiums, cafeterias, nightclubs, internet cafés, hotels, trade shows, pubs, casinos, communal areas, and waiting zones where mass events are held, among others, including cultural and sports venues.
The Manual shall enter into force on October 8, 2025, and the main change is that the expression "100% smoke-free environments" is expanded to include "and aerosols emitted by substitutes and imitators", thereby expressly covering electronic cigarettes and vaping devices, which must now be signaled as follows:

The second regulation was Resolution No. 30838 of May 22, 2025, issued by the Superintendence of Industry and Commerce.
The Superintendence of Industry and Commerce regulates the consumer's right to access information regarding cigarettes, tobacco products, their derivatives, substitutes or imitators, and the devices required for their operation.
The regulation establishes a minimum set of information that must be provided, including:
- Instructions for use.
- Product lifespan.
- Safety warnings, as well as instructions for storage, preservation, and proper use.
- Clear and precise assembly instructions for the devices.
- Identification of included accessories.
- Type of battery, power specifications, safety recommendations, and warnings related to battery use.
- Instructions for device maintenance.
- Warranty period.
This information must be presented clearly, in legible characters, in writing, and in Spanish, and must be included either on the product's label, packaging, container, or accompanying documentation.

The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.


