- within Media, Telecoms, IT and Entertainment topic(s)
- in Turkey
The decision is from an Opposition appeal. The invention relates to a method for estimating glomerular filtration rate (GFR) for assessing animal kidney function. The Opponent filed an appeal alleging that the distinguishing feature—multiplying the two concentrations—is merely a mathematical operation, which is non-technical.
However, the Board agreed with the patentee and acknowledged that while the method involves a mathematical operation, it is applied to biological measurements (SDMA and sCr), which are technical data. The combination of these two biomarkers leads to an improvement in GFR estimation.
Here are the practical takeaways from the decision: T 1423/22 (Renal disease/IDEXX) June 23, 2024, of the Technical Board of Appeal 3.3.08.
Key takeaways
All of the features that contribute to that technical character, i.e. that solve a technical problem by providing a technical effect, are to be taken into account in the assessment of inventive step, even if these features are non-technical per se.
Estimating the GFR, a clinical parameter relevant in renal diseases, based on measuring the blood concentrations of two markers (SDMA and sCr) is a technical problem
A non-technical calculation step can also contribute to solving a technical problem, together with other measurement steps.
The invention
The invention relates to a method for estimating glomerular filtration rate (GFR), which is crucial for assessing animal kidney function. The method involves measuring two biomarkers from blood samples: symmetrical dimethylarginine (SDMA) and creatinine (sCr). These concentrations are then multiplied, and the resulting product is compared to species-specific standard values to estimate GFR more accurately. This approach seeks to improve the precision of GFR estimation, which is important for diagnosing renal diseases in animals.
Claim 1 of Main Request (itemisation of the features added by the board)
A method for estimating glomerular filtration (GFR) rate in an animal subject, the method comprising:
measuring the concentration of free symmetrical dimethylarginine (SDMA) in a blood sample from the subject;
measuring the concentration of creatinine in a blood sample from the subject; and
comparing a value resulting from an equation comprising the product of the concentration of creatinine and the concentration of free SDMA to one or more standard values that correlate to glomerular filtration rate in the animal subject.
Is it patentable?
The Board identified the distinguishing features and comparison of technical and non-technical features as follows:
11. The claim concerns a method for estimating the GFR in an animal subject and comprises the technical steps of measuring the concentration of free SDMA and that of sCr in a blood sample from the subject ("step (i)" and "step (ii)"), and a step of "comparing a value resulting from an equation comprising the product of the concentration of creatinine and the concentration of SDMA to one or more standard values that correlate to glomerular filtration rate in the animal subject" ("step (iii)").
12. Step (iii) of the claimed method hence relates to a mathematical operation (multiplication of two measured values together) and to a mental act (comparing the value of the resulting product to one or more standard values), i.e. is non-technical. The claim therefore consists of a mixture of technical and non-technical features...
14. Document D1 discloses measuring concentration levels of both sCr and SDMA in dogs and demonstrates their correlation with the GFR measured by iohexol clearance (see the figure and the second to fourth paragraphs). The document concludes that SDMA "correlates well with GFR ... and might be a useful addition to sCr in the assessment of renal function" (see the last paragraph).
15. The claimed method differs from that proposed in document D1 in step (iii) (see point 11. above), i.e. in that a value resulting from an equation comprising the product of the sCr and SDMA concentration values is compared to one or more standard values that correlate to the GFR in the animal subject. This was not contested.
The Opponent argued that the distinguishing feature was not technical, an thus the subject-matter lacked inventive step.
16. However, the appellant contested that this distinguishing feature, which was non-technical, contributed to the technical character of the claimed method.
17. If a claimed invention consists of a mixture of technical and non-technical features, as is the case with the present claim 1 (see points 11. and 12. above), and has a technical character, i.e. solves a technical problem, all of the features that contribute to that technical character, i.e. that solve a technical problem by providing a technical effect, are to be taken into account in the assessment of inventive step, even if these features are non-technical per se (T 641/00, Headnote 1, and Reasons 5 and 6; G 1/19, OJ EPO 2021, A77, Reasons 140).
18. The claimed method has a technical character as it solves the technical problem of estimating the GFR, a clinical parameter relevant in renal diseases, based on measuring the blood concentrations of two markers (SDMA and sCr). Step (iii), which is non-technical, contributes to solving this technical problem, together with measurement steps (i) and (ii), because the GFR estimated for an animal subject is determined by the recited calculation of a product of measured SDMA and sCr concentration values and a comparison of this product to one or more standard values that correlate to the GFR in the animal subject.
19. It is noted that the claim lacks an explicit link of step (iii) to the actual estimation of the GFR as it does not recite that by comparing the product of the sCr and SDMA concentration values to one or more standard values that correlate to the GFR in the animal subject the value of the correlated GFR is read out, and that this correlated GFR represents the GFR estimated for the animal subject. However, despite this deficiency, the skilled person immediately understands from the wording of the claim that the comparison to standard values, which correlate to a particular GFR, directly and necessarily leads to the estimation of the GFR in the animal subject for which the blood markers were measured. The missing explicit link of how the steps of the claimed method result in the estimation of the GFR can thus be implicitly understood from the method steps.
20. Indeed, if the claimed method were to be interpreted as nothing more than a mere comparison of the product of the measured sCr and SDMA concentration values to one or more standard values that correlate with the GFR in the animal subject, without linking this comparison to the estimated GFR in the animal subject, the purpose of the claimed method – estimating the GFR in an animal subject – would not be achieved.
21. However, since this purpose of the claimed method is expressed in the claim as a functional feature, not achieving this effect by the steps of the claimed method would result in a lack of sufficiency of disclosure. Yet no objection on sufficiency of disclosure was raised by the appellant (see also section II. above).
22. Contrary to the appellant's view, step (iii) hence contributes, together with the technical steps (i) and (ii) of measuring the blood sCr and SDMA concentrations, to providing a method for estimating the clinical parameter GFR in an animal subject and hence contributes to the technical character of the claimed method.
The Board disagreed with the Opponent, and reasoned as follows:
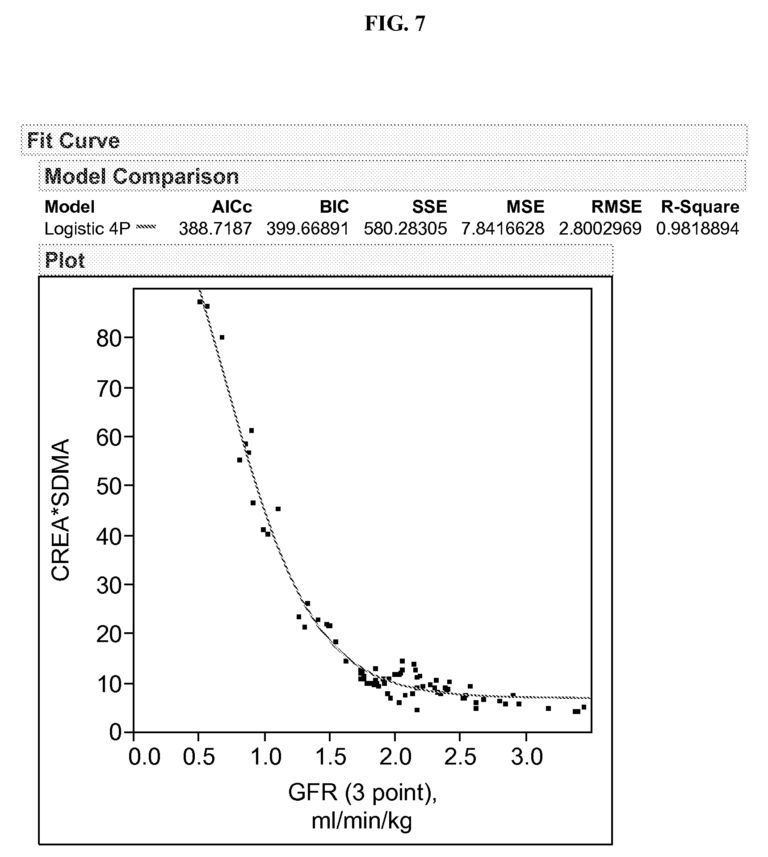
23. According to the patent, the product of the concentration values of sCr and SDMA allows for an improved estimation of the GFR compared to either concentration value alone (see paragraphs [0143] and [0148], Examples 6 and 7, and Figures 7 and 11 of the patent). However, as document D1 already suggests measuring the concentration values of both sCr and SDMA for the assessment of renal function (see the last paragraph of document D1 and point 14. above), an improvement over the method proposed in document D1 cannot be acknowledged.
24. The objective technical problem is therefore the provision of an alternative method for estimating the GFR.
25. The appellant contested that the objective technical problem could be formulated in this manner. The claim required that the standard values must be obtained in the same (individual) animal subject for which the GFR was to be estimated by the same measurements and calculations, which resulted in a "circular mathematical reasoning". According to the appellant, the objective technical problem was thus the provision of an alternative way of mathematically treating results obtained from measurements of blood samples from animal subjects.
26. The appellant's claim construction cannot be followed however. The expression "standard values that correlate to glomerular filtration rate in the animal subject" defines that these values are applicable to the animal subject for which the GFR is to be estimated, but not that these standard values have to be determined, first, in the same (individual) animal subject.
Therefore, the Board decided that since none of the appellant's arguments as to why the claimed method was obvious for the assessment of the inventive step were persuasive, the the Board rejected the appeal of the Opponent and maintained the patent as amended.
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.


