The inclusion of a disclaimer, where subject matter is carved out from the scope of a patent claim by means of a negative feature is not explicitly allowed for in the European Patent Convention (EPC). However, practice has evolved to allow claims to be amended to include a negative limitation in some circumstances. G1/16 is a new referral to the EPO's Enlarged Board of Appeal seeking clarification on when the inclusion of a disclaimer adds additional information not present in the application as filed in contravention of Article 123(2) EPC.
"Undisclosed Disclaimers" and the G1/03 Criteria
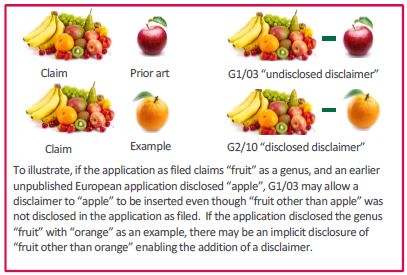
G1/03 dealt with the situation of "undisclosed disclaimers", those which seek to carve out from the scope of a claim subject matter of an earlier disclosure which the applicant could not have been aware of at the time of filing or subject matter that could not be covered for non-technical reasons, e.g. because it relates to unpatentable subject matter. G1/03 set out strict criteria regarding when such "undisclosed disclaimers" were permissible. That decision has been interpreted as exempting disclaimers that meet the criteria from the need to be derived from the application as filed in order to comply with the requirements of Article 123(2) EPC.
G2/10: "Disclosed Disclaimers" derivable from the application
G2/10 dealt with the situation where subject matter disclosed in an application, e.g. as a possible embodiment, is removed from the scope of the claim. Such disclaimers are only deemed permissible where the remaining subject matter of the resulting claim is derivable from the application as filed, e.g. because the skilled person would have understood that the subject matter lacking the embodiment disclaimed is a possible alternative.
Diverging approaches
The Board of Appeal (Board 3.3.09) in referring case T437/14 was of the view that G2/10 requires that all amendments without exception, including one to insert an "undisclosed disclaimer", must meet the requirements of Article 123(2) EPC restricting the inclusion of new, undisclosed, subject matter. The present Board considered that the reasoning of the Enlarged Board in reaching its decision in G1/03 is based on a false assumption, and the criteria set out in that case are now superseded by G2/10. Therefore, a claim to "fruit other than apple" would be considered to add matter in contravention of Article 123(2) EPC because the remaining subject matter of the resulting claim is not derivable from the application as filed.
However, as the Enlarged Board in G2/10 did not explicitly set aside G1/03, a question remains as to whether the two different tests are valid alternatives. Many Opposition Divisions consider that if the criteria of the "undisclosed disclaimer" test in G1/03 are met, then the amendment is allowable and the requirements of Article 123(2) EPC do not need to be considered further, a position endorsed in the EPO's Guidelines for Examination. Furthermore, some Boards of Appeal apply a relaxed interpretation of the requirements of Article 123(2) EPC when considering "undisclosed disclaimers" that meet the criteria laid down in G1/03, assessing whether the technical teaching is modified after the disclaimer has been introduced. Under this approach, disclaimers that merely slightly reduce the scope of a claim without materially affecting the generic teaching can be allowed.
For example, if the technical teaching is that fruit is that it provides a source of dietary vitamins, a reduced claim directed to "fruit other than apple" does not affect that teaching and the slightly reduced scope remains allowable.
Questions referred to the Enlarged Board in G1/16
The Board of Appeal considers the divergent approaches raise fundamental questions of law and has for referred the following questions [paraphrased] to the Enlarged Board of Appeal:
Is the standard referred to in G2/10 for the allowability of disclosed disclaimers under Article 123(2) EPC also to be applied to claims containing undisclosed disclaimers?
If the answer to the first question is yes, is G1/03 set aside as regards the exceptions relating to undisclosed disclaimers?
If the answer to the second question is no, is the standard referred to in G2/10 under Article 123(2) EPC modified in view of the exceptions relating to undisclosed disclaimers defined in G 1/03?
Impact of the decision
The answers given by the Enlarged Board will have a significant impact on the allowability of disclaimers and the ability of an applicant to carve out subject matter after filing. A decision answering "yes" to questions 1 and 2, setting aside G1/03 will have a particularly profound effect on applicants in the life sciences area as it will curb the ability of an applicant to disclaim subject matter considered patentable for non-technical reasons, such as methods of treatment or biological processes. We will report further when the decision of this referral is available.
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.

