- within Food, Drugs, Healthcare and Life Sciences topic(s)
- INTRODUCTION
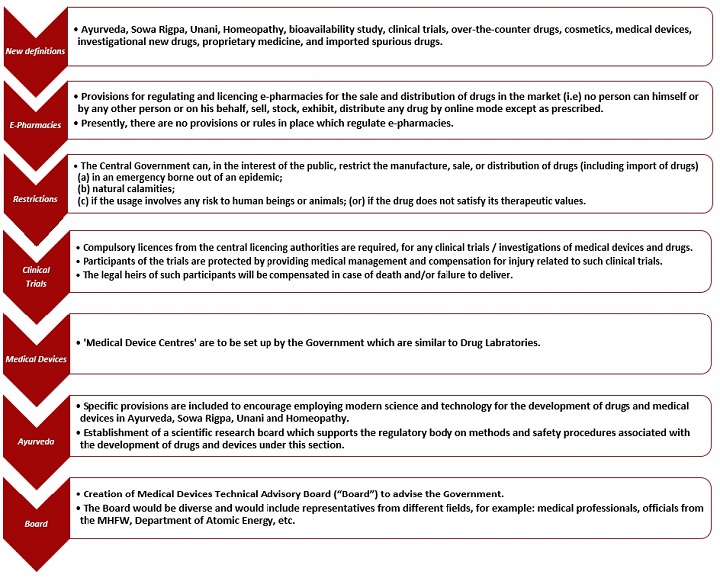
The Ministry of Health and Family Welfare ("MHFW"), on 8 July 2022, released a draft of the Drugs, Medical Devices and Cosmetics Bill, 2022 ("Bill") for objections, comments, and suggestions from the public and from relevant stakeholders.1 The Bill will replace the existing Drugs and Cosmetics Act, 1940 ("Act"). The provisions of the new regulation will be in addition to, and not in derogation of any other law for the time being in force. The Bill comprises regulations on online pharmacies, clinical testing, medical devices, Ayurveda, Sowa Rigpa, Unani, and Homeopathy, and their respective advisory boards.
- KEY CHANGES FROM THE ACT
- PENALTIES
The Bill includes penalties for multiple offences in the form of imprisonment and fines of varied nature. Some of the key provisions are:

- COMMENTS AND ISSUES ON THE BILL
- The definition of 'clinical trial' significantly deviates from World Health Organization ("WHO") standards and has been left extremely vague and open to interpretation. As per WHO, 'clinical trial' refers to "any research study that prospectively assigns human participation or groups of humans to one or more health-related interventions to evaluate the effects on health outcomes." This definition includes Interventional Trials as well as Phase I to Phase IV trials.
- Good manufacturing practices, which are the standard and acceptable criteria, have not been covered.
- The bill fails to provide any regulation on post-marketing surveillance for medical devices. In practice, implants tend to remain within patients' bodies for years and have long term harmful effects.
- Further, the bill lacks provisions for implementing mandates for companies to recall devices or medicines in case of issues concerning such devices and medicines.
- Lastly, the bill only provides a recommendation to the Government to formulate regulations for the operation of e-pharmacies but fails to provide any guidelines for the same.
- CONCLUSION
The implementation of the Bill by the Government is long overdue. There has been increasing demand from medical professionals and policymakers calling for legislation that takes the current conflict and technology into context by bridging the lacunae in law. Yet, the Bill remains vague and requires clarification by the Government, which hopefully will be provided prior to its implementation.
Footnote
1 Drugs, Medical Devices and Cosmetics Bill, 2022, Accessed at: https://main.mohfw.gov.in/newshighlights-97 .
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.




