- within Intellectual Property topic(s)
- in United States
- with readers working within the Pharmaceuticals & BioTech industries
- with readers working within the Pharmaceuticals & BioTech industries
- within Compliance topic(s)
China has developed rapidly in the pharmaceutical industry in the past few years and has become one of the largest and most active pharmaceutical markets in the world. To further encourage innovation in drug development, China has initiated a series of regulatory and patent reforms in the pharmaceutical industry in recent years, including the implementation of patent term extension (PTE).
Background
The pharmaceutical industry is highly dependent on patent protection due to the high cost, long cycle and high risk of researching and developing new drugs. Without patent protection, it would be difficult for innovative drug companies to recover their research and development costs and achieve reasonable profits, thereby negatively affecting their enthusiasm for research and development of new drugs. However, drug marketing requires strict approval by the drug regulatory authorities, including a series of studies like non-clinical safety evaluation and clinical trials. This means that even if a patent application is granted, it cannot be implemented for a considerably long period until it obtains a marketing approval, resulting in a greatly shortened protection period of the patent.
Patent term extension originated in the United States under Drug Price Competition and Patent Term Restoration Act in 1984, also known as the Hatch-Waxman Act. Then, several countries or regions, such as Japan, South Korea, the European Union and Canada have also established similar systems to compensate for the loss of patent protection period caused by the lengthy approval process for innovative drugs.
China has been exploring the patent term extension in recent years. On 15 January 2020, China agreed to provide patent term extension to compensate for unreasonable delays that occur in granting the patent or during pharmaceutical product marketing approvals under the Economic and Trade Agreement between the Government of the People's Republic of China and the Government of the United States of America. On 17 October 2020, China passed the amendments to the Chinese Patent Law. Since 1 June 2021, the new Chinese Patent Law has come into effect and patent term extension has been available in China. The new Implementing Regulations and Patent Examination Guidelines came into effect on 20 January 2024 and further detailed the implementation of PTE in China.
Overview of PTE
The new Chinese Patent Law stipulates in paragraph 3 of article 42 that to compensate for the time taken for review and approval of a new drug for marketing, the China National Intellectual Property Administration (CNIPA) will extend the term of the patent for invention related to the new drug for which a marketing approval is obtained in China, at the request of the patentee. The patent term extension should not exceed five years and the resulting total effective patent term should not exceed 14 years from the approval for marketing of the new drug.
- Eligible drugs of PTE
PTE is available to product patents, preparation method patents and medical use patents of the active pharmaceutical ingredient (API) contained in a 'new drug'. The term'new drug' here is interpreted to mean innovative drugs and certain improved new drugs defined in drug regulatory laws and provisions of the National Medical Products Administration (NMPA). For a pharmaceutical product to qualify as a 'new drug' that is eligible for PTE in China, the pharmaceutical product must be new to the world, meaning that the new drug application (NDA) of the product must be filed in China before the product is approved for marketing in any other countries. However, it is not a requirement for the NDA to be approved in China before it is in other countries.
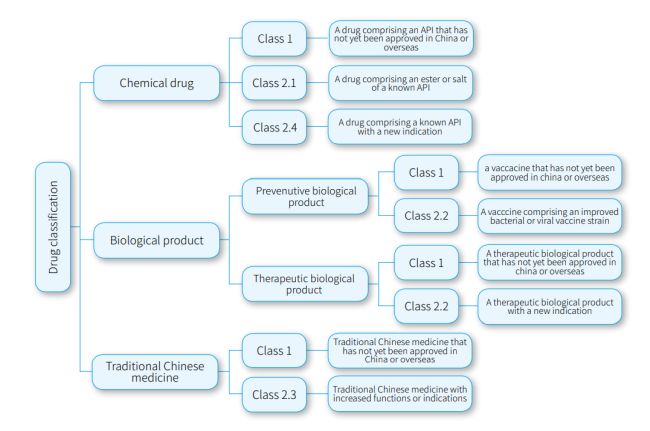
- Innovative drugs eligible for PTE: according to the current drug classification provisions of NMPA, innovative drugs refer to class 1 drugs and must be new to the world. Innovative drugs include the following drugs that have not yet been approved in China or overseas: (i) a chemical drug; (ii) a preventive biological product – vaccine; (iii) a therapeutic biological product; and (iv) traditional Chinese medicine.
- Improved new drugs eligible for PTE: improved new drugs
eligible for PTE must be new to the world as well and are limited
to the following drugs according to the NMPA's drug
classification:
- an ester or salt of a known API in Class 2.1 of chemical drugs;
- a drug comprising a known API with a new indication in class 2.4 of chemical drugs;
- a vaccine comprising an improved bacterial or viral vaccine strain in class 2.2 of preventive biological products;
- a therapeutic biological product with a new indication in class 2.2 of therapeutic biological products; and
- traditional Chinese medicine with increased functions or indications in class 2.3 of traditional Chinese medicine.
- 'Imported drugs' are not eligible: according to the above definition of 'new drug', the 'imported drugs' in class 5.1 of chemical drugs, classes 3.1 and 3.2 of preventive biological products and classes 3.1 and 3.2 of therapeutic biological products, which have been marketed overseas when seeking marketing approval in China, are excluded from PTE.
Drug classification eligible for PTE is summarized in the following chart.
- Time limit for filing of a PTE request
A PTE request should be filed by the patentee or its agent upon authorization of the marketing authorization holder within three months from the drug's approval date, together with the payment of the official fee.
- Requirements for obtaining a PTE
To obtain a PTE in China, the following requirements should be met:
- the issue date of the patent shall be earlier than the approval date of the drug;
- the patent is valid when the PTE request is filed;
- the patent has not been granted a PTE yet;
- the claims of the patent include the drug-related technical solutions; the drug-related technical solutions refer to the structure, components and amount thereof and the approved manufacturing process and indications of the approved new drug;
- if multiple patents cover the drug, the term of only one patent can be extended; and
- if a patent covers multiple drugs, PTE of the patent is
possible for one drug only.
- Calculation of PTE
PTE is applied in addition to any patent term adjustment (PTA) and the calculation of PTE shall be done after the decision of PTA is made. Due to different reasons for compensation, the compensation periods involving PTA and PTE can be accumulated.
PTE is calculated by subtracting five years from the number of days between the filing date of the Chinese patent application and the marketing approval date in China. Meanwhile, PTE should meet the following two requirements: the maximum PTE is five years and the resulting total effective patent term after drug approval for marketing should not exceed 14 years.
The following line chart and equations show the two requirements regarding the extension period for PTE.
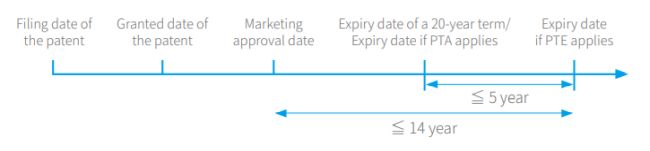
PTE = (marketing approval date in China – filing date of the Chinese patent application) five years (≦5 years)
Total effective patent term after drug approval for marketing = (the expiry date of a 20-year patent term – marketing approval date in China) + PTA (if any) +PTE (≦14 years)
- Examination of the PTE request and challenge to CNIPA's decision
Before making an unfavorable decision, the examiner will give the patentee at least one opportunity to make observations or amendments. If PTE requirements are met, the CNIPA will grant PTE, notify the number of days by which the patent term is extended and publish information such as drug name, approved indications and the original and new expiry dates of the patent in the patent gazette.
The CNIPA's decision is challengeable by the patentee and an interested third party by applying for an administrative reconsideration at the CNIPA.
- Scope of protection during PTE
Since the purpose of PTE is to compensate for the time taken for the market approval of the new drug, it is necessary to directly associate the approved new drug with the technical solutions of the drug patent that requests PTE. The technical solutions related to a new drug would serve as the bridge connecting the marketed new drug with the drug patent that requests PTE.
The protection scope of PTE is narrower than the patent and limited to the new drug for the indication as approved, which is interpreted to mean that the protection scope of PTE is: (i) new drug product used for the approved indications for product claims; (ii) the approved indication of the new drug for medical use claims; and (iii) manufacturing process recorded with NMPA for the new drug used for the approved indications for preparation method claims.
A new indication can support a new PTE, but the scope of PTE obtained only covers the new indication underlying the PTE but not any earlier or later approved indications.
Therefore, the patentee when filing a request for PTE, should provide materials for determining the protection scope of PTE, such as materials showing the composition of the approved drug, materials showing the indication of the approved drug, materials showing the drug manufacturing process approved by NMPA and the like.
Takeaways
For innovative drug companies, due to the lengthy process from the discovery of 'hit' compounds, to the optimization of lead compounds, to preclinical and clinical trials of candidate drugs and to marketing, patent applications are often filed long before the drug is marketed. The implementation of PTE in China has positive effects for innovative drug companies, since it can provide extended protection of patents of innovative drug companies, if PTE is granted by the CNIPA. Under PTE, the protection of the legitimate rights and interests of innovative drug companies is strengthened to incentivize continuous innovation in new drug research and development.
Therefore, the innovative drug companies should actively seek for extension of the patent term by utilizing PTE in China. When requesting for PTE, the innovative drug companies should take into account all the factors, such as the type of the claims, scope of the claims, stability of the patent, enforcement of patent, PTE scope and 'one PTE per drug per patent' rule so as to maximize and rationalize the extension of the patent term of one or more patents. For example, the patentee may choose one patent to file a PTE request based on the new drug and its approved indication and then choose another patent to file a PTE request based on a newly approved indication. Therefore, patents filing strategy may also need to adapt to the NDA approvals such as indication expansion.
As mentioned earlier, imported drugs that have been marketed overseas when seeking marketing approval in China, including class 5.1 of chemical drugs, classes 3.1 and 3.2 of preventive biological products and classes 3.1 and 3.2 of therapeutic biological products, are not eligible for PTE. Therefore, innovative drug companies should consider submitting the NDA in China before the new drug is marketed in other countries.
For generic drug companies, extending the patent term of innovative drugs will inevitably delay the entry of generic drugs into the market, resulting in a significant impact on numerous generic drug companies in China. The generic drug companies should closely monitor the patent term of innovative drug companies, pay attention to whether a PTE has been obtained and whether CNIPA's decision for PTE is challengeable and plan for the Abbreviated New Drug Application (ANDA) progress strategically. If necessary, the generic drug companies may also consider filing a request of invalidation against the patents of innovative drug companies.
The resulting delayed entry of generic drugs into the market is likely to increase patients' pharmaceutical expenses in China. Therefore, seeking for a balance between the profit of innovative drug companies and generic drug companies is significant. When formulating patent laws and implementing regulations regarding PTE, this has already been taken into consideration and could be reflected in several requirements for obtaining a PTE. For example, the requirements include that only patents for which a PTE has not been granted are eligible to receive a PTE; if the drug is covered by multiple patents, the term of only one patent can be extended; and if a patent covers multiple drugs, PTE of the patent is possible for one drug only. Besides, it is also stipulated that the maximum PTE is five years and the resulting total effective patent term after drug approval for marketing shall not exceed 14 years. These requirements not only effectively avoid repeated compensation of the patent term of innovative drug companies, but also prevent excessive extension of a patent term.
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.

