- with readers working within the Advertising & Public Relations industries
- with Senior Company Executives and HR
PatentNext Summary: The life sciences and healthcare fields produce big data, which Artificial Intelligence (AI) tools can use to train AI models helpful in providing doctors, patients, researchers, and other stakeholders with various benefits. In the intellectual property (IP) space, there has been explosive growth in this area, with AI-based patent filings addressing trending areas, including disease identification and diagnosis, drug discovery, personalized medicine, and clinical trials, among others. These trends are expected to continue though challenges remain, such as the balance between the need for health-related data to train AI models and privacy and ethical concerns in using such data.
The fields of life sciences and healthcare generate vast quantities of data, e.g., "big data," which can come from various sources, including hospitals, doctors, patients, caregivers, and researchers.
Artificial Intelligence (AI), in particular, machine learning (ML), thrives on big data for training accurate AI models. Because of this, AI lends itself to an apt new tool that complements the vast quantities of data typically generated by the life sciences and healthcare fields.
For example, once trained on the data generated in these fields, AI models may be used to develop innovations that can address disease identification, diagnosis, treatment, and drug discovery, among other trending areas.
Several trends have emerged for use of AI as a tool in the life sciences and healthcare industries. The below provides a non-exhaustive list of emerging trends.
1. Disease Identification and Diagnosis
The Centers for Disease Control and Prevention estimates that six in ten adults in the U.S. have a chronic disease, and four in ten adults have two or more. By 2025, it is estimated that chronic diseases may affect 164 million Americans – nearly half (49%) of the U.S. population.
To combat this, researchers have used AI as a tool to identify and diagnose chronic diseases. For example, the Mayo Clinic announced three instances of applying AI in the field of cardiovascular medicine:
- Mitigating stroke impact. In emergency rooms, when people come in with a stroke called an intracerebral hemorrhage, they get a CT scan. That scan is examined by a computer trained to analyze CT data, cutting the time to diagnosis and limiting brain damage.
- Preventing heart problems. Applying AI to electrocardiograms (ECGs) has resulted in a low-cost test that can be widely used to detect the presence of a weak heart pump, which can lead to heart failure if left untreated. The Mayo Clinic has a database of more than 7 million ECG records. The Mayo Client removes all identifying patient information to protect privacy. Then, the resulting data is mined to accurately predict heart failure noninvasively, inexpensively, and within seconds.
- Detecting atrial fibrillation (a-fib) sooner. AI-guided ECGs are also used to detect faulty heart rhythms (atrial fibrillation) before any symptoms are evident.
The May Clinic announced that these AI-based tools have moved from the research stage to use in the clinic.
2. Drug Discovery and Manufacture
A machine learning model can be trained on pharmaceutical, bio, and other life sciences and/or healthcare data to aid researchers with early-stage drug discovery, including the development of new drug compounds and the development of discovery technologies to assist with next-generation sequencing.
For example, Nvidia released an AI framework ( Nvidia BioNeMo) for training and deploying large biomolecular language models. Nvidia BioNeMo uses a large language model (LLM) framework and supports chemistry, protein, DNA, and RNA data formats. In particular, Nvidia BioNeMo includes four pre-trained language models accessible in the cloud. The models can be used by researchers to analyze amino acid sequences for predicting protein properties, protein modeling, reaction prediction, molecular optimization, and de novo molecular generation.
This allows researchers and scientists to train large-scale language AI models on big data datasets, resulting in better-performing AI models (e.g., neural networks), and, ultimately, allows scientists better understand diseases and find therapies for patients.
3. Personalized Medicine
AI models can be trained to provide customized treatment based on a person's unique health history. Such models can provide optimized diagnoses and treatment protocols on a patient-specific level.
For example, at Johns Hopkins University, researchers use whole-heart computational AI models to better understand ventricular arrhythmias. These models included specific biophysical complexity of an individual patient's cardiac pathology, factoring in cellular- and organ-level properties.
According to Natalia Trayanova, professor of biomedical engineering and medicine at Johns Hopkins University, this complex biophysical system "can be represented using a set of mathematical equations," said Natalia A. Trayanova, a co-author and professor of biomedical engineering and medicine at Johns Hopkins University. "Solving these equations using computer software allows us to run detailed simulations to mimic the heart's electrical activity."
"Personalized computational modeling of patient hearts is making strides in developing models that incorporate the individual geometry and structure of the heart, as well as other patient-specific information," Trayanova said in a press release.
"These types of models can enable fast evaluation of medical device settings and patient-selection criteria, as well as the development of novel therapeutic agents," she explained.
4. Clinical Trials
The National Library of Medicine (NLM) acknowledges that AI could be used to modernize the crucial steps of clinical trial conduct-study design, planning, and execution. For example, AI can be used for linking big data, including electronic medical records (EMRs), published medical literature, and clinical trial databases, in order to improve recruitment by matching patient characteristics to selection criteria.
For example, according to the NLM, AI can assist by enhancing patient selection in the following ways:
- By Reducing population heterogeneity by harmonization of large EMR data from diverse formats and different levels of accuracy and by leveraging electronic phenotyping.
- By prognostic enrichment, which would include selecting patients who have a higher probability of having a measurable clinical endpoint. For example, ML techniques using key biomarkers of Alzheimer's disease (AD) have been deployed for prognostic enrichment.
- By predictive enrichment, which can include choosing a population with a better likelihood of responding to treatment. For early AD, a clinical trial simulation tool developed by modeling drug, disease, and progression of disease, which helped in predictive enrichment, has undergone regulatory review.
In addition, AI systems can be utilized for automatic analysis of EMR and clinical trial digital eligibility databases and match these with recruiting clinical trials from trial announcements, social media, or registries.
It can also help patients become aware of clinical trials of interest sooner and allow them to approach investigator sites for evaluation of eligibility. According to the NLM, AI-based clinical trial matching has facilitated an increase in enrollment in a lung cancer trial by 58.4%.
5. Radiotherapy and Radiology
The National Library of Medicine (NLM) has also reported on the topic of AI in radiotherapy, where AI can be applied to images (e.g., MRI CT scan, or ultrasound images) to segment and identity parts of the body, such as bones, organs, muscles, and fractures. The segmented areas may reveal portions of the body needing radiotherapy or radiology treatment.
For example, according to the NLM, breast cancer is one of the most commonly diagnosed cancers, responsible for 30% of all new cancer diagnoses in women. Ultrasound is normally used to diagnose breast cancer, and improvement in segmentation of breast ultrasound images into functional tissues provides a better tumor localization, assessment of treatment response, and breast density measurement.
Typically, segmentation of ultrasound is very time-consuming for radiologists and it is skill and experience-dependent. However, AI can be used to provide automated segmentation of ultrasound images, which can help mitigate those problems.
According to the NLM, a recent study shows convolutional neural network (CNN)-based segmentation can segment the 3D image into four major tissues: skin, mass, fibro glandular tissue, and fatty tissue with high accuracy. Such use of AI provides segmentation in the future in clinical diagnosis of breast cancer.
6. Electronic Health Records (EMR)
EMR data constitutes big data for training AI models. The National Library of Medicine (NLM) reports a need to correlate EMR data to provide a base for training AI models. See Prospect of Artificial Intelligence Based on Electronic Medical Record.
According to the NLM, AI data management software can be expected to increase the efficiency of hospital operations, patient management, and treatment.
Through AI, it is possible to classify diseases accurately, reclassify preexisting disease categories according to individual characteristics, quickly analyze images and medical data in EMR, and provide appropriate services. With the emergence of medical AI-integrated platforms, AI has become essential for the creation of new services, such as the improvement of medical quality and real-time health management.
Patenting Trends for inventions using AI in the Life Sciences and healthcare Fields
Patent filings at the United States Patent and Trademark Office (USPTO) across the above-referenced life science and healthcare categories reflect a similar trend.
The below chart shows AI and Life Science patent filings by Technology ("Tech") Center over time from 2000 to 2022, where we see a spike in activity in post-2016.
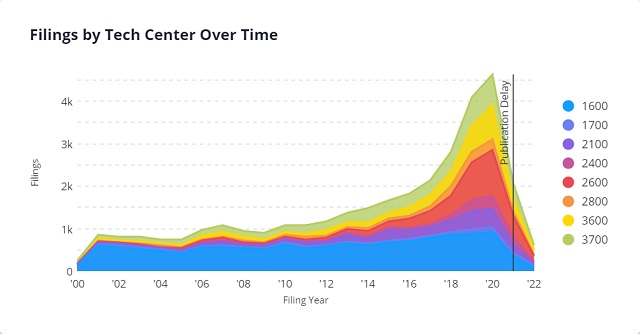
Most of the patent filings come from technology companies focusing on AI-related technologies. These include IBM, Nvidia, and LG Electronics.
After the spike in 2016, the above chart shows continued activity up to the present day. Given the increased interest in AI and the life sciences and healthcare fields, we can expect further filings in this space in the coming years (note that the right-most side of the graph slopes down because of the 18-month "Publication Delay," during which information for newer patent application filings, is not yet publicly available. See 37 CFR § 1.211).
In addition, the above chart organizes patent application filings by USPTO Tech Centers. As shown, most AI-based life science/healthcare patent applications fall into Tech Center 1600, which focuses on Biotechnology and Organic Chemistry. We can see that this tech center has handled a majority of AI-based life science and healthcare applications since 2000.
However, since 2019, Tech Center 2600, which focuses on Communications, has received most AI-based life science/healthcare patent applications. This tech center includes art units that focus on computer graphic processing ( art unit 2615) and image analysis ( art unit 2660), which are important to radiotherapy and radiology inventions.
The FDA's Artificial Intelligence and Machine Learning Action Plan
Innovators of AI-based life science and healthcare inventions should familiarize themselves with the regulatory landscape, including the Food and Drug Administration (FDA)'s position on Artificial Intelligence (AI) and machine learning (ML) based software inventions.
The FDA has identified AI as an important technology that it will monitor and regulate, stating that: "Interest in medical devices incorporating ML functionality has increased in recent years. Over the past decade, the FDA has reviewed and authorized a growing number of devices legally marketed (via 510(k) clearance, granted De Novo request, or approved PMA) with [machine learning] across many different fields of medicine—and expects this trend to continue."
Accordingly, any new AI technology, which proposes to improve the efficiency of clinical trial design and conduct, should be validated by testing alongside the existing technology it claims to complement or substitute.
The FDA maintains a list of AI/ML-enabled Medical devices submitted to the FDA. The list includes devices submitted and authorized via 510(k) clearance, granted De Novo request, or an approved Premarket Approval Application (PMA).
The below chart summarizes the number of AI/ML submissions made to the FDA through the years.
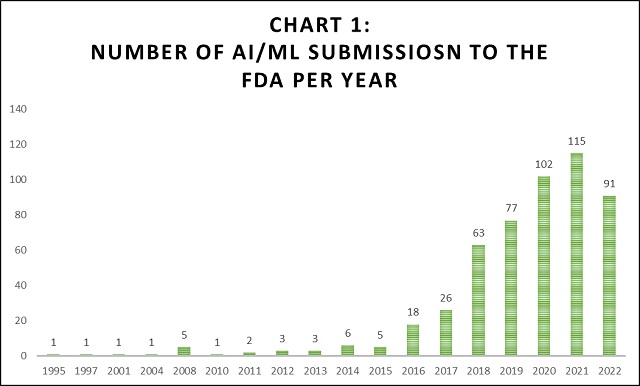
As shown in Chart 1 above, AI/ML submissions started increasing exponentially around the 2016 timeframe. This is correlates with AI and Life Science patent filings, as shown above.
By far, the Radiogoloy FDA panel received the most submissions (392 submissions) compared to any other group. The Cardiovascular FDA panel received the second most submissions (57 submissions). The remaining panels each received 15 or fewer submissions.
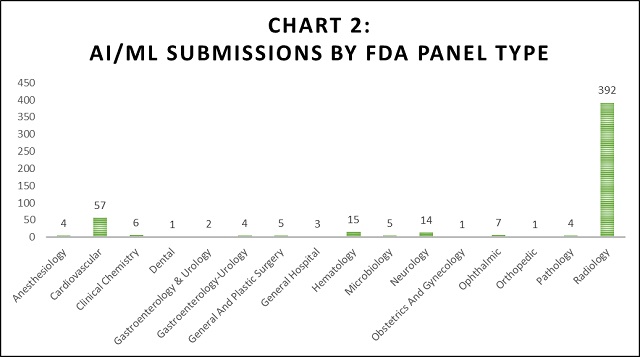
The FDA considers AI/ML-based software as a medical device. Because of this, the FDA expects AI innovators to comply with requirements of clinical, analytical, and technical validation, quality systems, good machine learning practice, assurance of safety and effectiveness, transparency, and real-world performance monitoring.
In order to address these issues and the increased submissions regarding Artificial Intelligence (AI) and Machine Learning (ML), the U.S. Food & Drug Administration (FDA) published an action plan outlining five actions the FDA intends to take. See FDA's AI/ML-Based Software as a Medical Device Action Plan (the "FDA's AI/ML Action Plan").
These five actions are summarized below.
- Developing a tailored Regulatory Framework for AI/ML-based devices.
The FDA categorizes AI/ML-based technologies as Software as a Medical Device (SaMD), which the FDA identifies as "software intended to be used for one or more medical purposes that perform these purposes without being part of a hardware medical device." Id. The FDA states that its vision for AI/ML-based SaMD is to provide "appropriately tailored total product lifecycle-based regulatory oversight to "deliver safe and effective software functionality that improves the quality of care that patients receive." Id.
- Harmonizing a set of Good Machine Learning Practices (GMLP)
The FDA published a paper on guidelines for Good Machine Learning Practices (GMLP). The GMLP includes a set of AL/ML best practices similar to software engineering best practices as typically applied in the software field.
The GMLP includes a set of ten guiding principles that the FDA will no doubt use to review AI/ML-based FDA submissions. As the FDA explains, "[t]hese guiding principles will help promote safe, effective, and high-quality medical devices that use artificial intelligence and machine learning (AI/ML)." Id.
The ten guiding principles of the GMLP are (1) leveraging multi-disciplinary expertise during the product lifecycle of the device; (2) ensuring the security of data through quality software engineering; (3) using training data representative of the intended patient population; (4) keeping training datasets independent of test data sets; (5) selecting reference datasets that are clinically relevant and well characterized; (6) ensuring the AI model generated from the training data reflect the intended use of the device; (7) keeping a "human in the loop" to use the AI/ML device; (8) testing the device in clinically relevant conditions; (9) the AI/ML device is used with patients for which the AI/ML model was trained, and such patients are provided information informing them of the same; and (10) monitoring AI/ML model, as deployed, to check for model accuracy and check for any need to update or retrain the model. Id.
- Patient-Centered Approach Incorporating Transparency to Users
One of the concerns of AL/ML devices has been a lack of transparency in the AI/ML models they use. For example, the FDA has identified unique challenges for manufacturers in clearly describing data to train an AI/ML model. This can include the relevance of its model inputs, the logic it employs (when possible), the role intended to be served by its output, and the evidence of the device's performance. Therefore, the FDA seeks to clarify its position on the transparency of AI/ML technology in medical device software in order to ensure that users understand the benefits, risks, and limitations of these devices and to support the transparency of and trust in AI/ML-based technologies.
- Regulatory Science Methods Related to Algorithm Bias & Robustness
Bias in AI/ML models poses a risk of alienating potential adopters of AI/ML-based technology. AI/ML-model bias is described by the FDA as follows: "healthcare delivery is known to vary by factors such as race, ethnicity, and socio-economic status; therefore, it is possible that biases present in our healthcare system may be inadvertently introduced into [a given AI/ML model]." FDA's AI/ML Action Plan.
Accordingly, any AI/ML model development should include steps for the identification and elimination of bias. This can include increasing data transparency (e.g., tracking and showing the data used to train a given AI/ML model), eliminating private information, eliminating biased data (e.g., race base data) from the model training sets, and/or including sufficient quantity and types of training data to train an AI/ML model to capture diverse populations for which an AI/ML device will be used.
- Real-World Performance (RWP)
Finally, the action plan encourages a "total product lifecycle" approach to AI/MLD devices. In this aspect, AI/ML devices output real-world data that is collected and monitored. Such real-world data "may allow manufacturers to understand how their products are being used, identify opportunities for improvements, and respond proactively to safety or usability concerns." FDA's AI/ML Action Plan.
Challenges Ahead
AI-based life science and healthcare trends are expected to continue though challenges remain. A major challenge comes from AI's need for big data for accurate, or at least useful, AI models to be produced. While big data is plentiful in life science and healthcare, tension arises with privacy and ethical concerns in using such data. For example, in several fields, the most useful information can often be personal medical data, which is difficult to access for a number of regulatory reasons, the Health Insurance Portability and Accountability Act (HIPAA) and General Data Protection Regulation (GDPR) being among a few of these.
However, efforts are underway to combat data privacy issues, including the collection and use of such data. For example, the White House's Office of Science and Technology Policy published the Blueprint for an AI Bill of Rights. The AI Bill of Rights explores ethical considerations when deploying AI. For example, according to the White House, the AI Bill of Rights is intended to "support the development of policies and practices that protect civil rights and promote democratic values in the building, deployment, and governance of automated systems." AI Bill of Rights, About this Document. See also Ethical Considerations of Artificial Intelligence (AI) and the White House's Blueprint for an AI Bill of Rights.
It may be that we see future regulation that addresses the use of healthcare-related data for developing AI-based innovations in the life sciences and healthcare fields.
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.
[View Source]

