Several patent litigations have been filed involving mRNA pioneers such as Moderna, Inc. and BioNTech, Inc. (with Pfizer) over the past year relating to sales of the Moderna and BioNTech/Pfizer COVID-19 vaccine products (Spikevax® and Comirnaty®, respectively). This post provides an overview of those cases, other cases involving other mRNA pioneers, and the overall mRNA patent litigation landscape as of August 24, 2022.1
Arbutus and Genevant v. Moderna
On February 28, 2022, Arbutus and Genevant sued Moderna in the U.S. District Court for the District of Delaware. The complaint alleges that Moderna infringed a number of Arbutus-owned patents (i.e., U.S. Patent Nos. 8,058,069, 8,492,359, 8,822,668, 9,364,435, 9,504,651, and 11,141,378) through, at least, Moderna's sale of its Spikevax® vaccine product.
The patents-in-suit are primarily directed to lipid nanoparticle (LNP) delivery technology, comprised of four lipids (i.e., cationic lipid, phospholipid, conjugated lipid, and cholesterol) designed to protect the fragile mRNA "payload" post-injection and release that payload once inside a recipient's cells. The complaint seeks monetary damages (including based on willful infringement) but does not seek an injunction.
Moderna and Arbutus previously litigated certain of Arbutus's LNP-based patents before the U.S.P.T.O., where Moderna challenged three of those patents through inter partes review (IPR) proceedings. As previously discussed, Moderna was successful in canceling all claims of U.S. Patent No. 9,404,127 and some claims of the '435 patent (but not all), and all claims of the '069 patent survived. As indicated above, the '435 and '069 patents are being asserted in the present case along with a number of other Arbutus patents.
On May 6, 2022, Moderna moved to dismiss the infringement claims directed to Moderna's sales to the U.S. government pursuant to 28 U.S.C. § 1498(a) (a statute allegedly requiring plaintiffs to seek redress for such infringing sales from the federal government in the Court of Federal Claims, rather than from Moderna), which plaintiffs have opposed. This motion does not appear to affect the allegations relating to Moderna's other (non-U.S. government) sales. In any case, the motion is fully briefed and the court recently ordered that the Rule 26(f) scheduling conference need not occur until after disposition of the pending motion to dismiss.
This case is before Judge Mitchell S. Goldberg and the caption for this case is: Arbutus Biopharma Corp. & Genevant Sciences GmbH v. Moderna, Inc. & ModernaTX, Inc., No. 1:22-cv-00252 (D. Del. filed February 28, 2022).
Alnylam v. Moderna and Pfizer
On March 17, 2022, Alnylam sued Moderna and Pfizer, separately, in the U.S. District Court for the District of Delaware. The complaints allege that each of Moderna and Pfizer infringed U.S. Patent No. 11,246,933 through at least their sales of Spikevax® and Comirnaty® vaccine products, respectively. The asserted '933 patent has claims directed to cationic lipids (one of the four lipid components that encapsulate an mRNA payload in commercialized lipid nanoparticles). The complaints seek monetary damages only (i.e., Alnylam is not seeking an injunction against any of the defendants).
As in the Arbutus case, Moderna moved to dismiss the claims directed to Moderna's sales to the U.S. government pursuant to 28 U.S.C. § 1498(a), which Alnylam has opposed. Interestingly, third parties Arbutus and Genevant are seeking leave of court to file an amicus brief in the Alnylam case with respect to the § 1498(a) issue, indicating the larger battle being waged involving the Arbutus LNP technology in Arbutus v. Moderna. These motions are fully briefed. As for Pfizer, it answered Alnylam's complaint and asserted counterclaims joined by BioNTech, bringing BioNTech into the case.
On July 12, 2022, as the originally-filed cases were pending, Alnylam again separately sued Moderna and Pfizer (and this time including BioNTech) in the District of Delaware, for infringement of newly granted U.S. Patent No. 11,382,979 (which issued the same day the suit was filed, is directed to an entire lipid nanoparticle, and is in the same patent family as the '933 patent). The March and July Moderna cases have been consolidated, as were the March and July Pfizer/BioNTech cases.
Each consolidated case is before Judge Colm F. Connolly who recently ordered that both scheduling conferences occur before the court on the same date and time unless there are no disputes between the parties with respect to scheduling, in which case a scheduling conference would not be necessary. If a scheduling conference is necessary for either or both of the consolidated cases, such scheduling conference has been ordered to occur on September 1, 2022 at 10:30 am.
The captions for these cases are: Alnylam Pharmaceuticals, Inc. v. Moderna, Inc., ModernaTX, Inc. & Moderna U.S., Inc., No. 1:22-cv-00335 (D. Del. filed Mar. 17, 2022); Alnylam Pharmaceuticals, Inc. v. Pfizer Inc. & Pharmacia & Upjohn Co. LLC, No. 1:99-mc-09999 (D. Del. filed Mar. 17, 2022); Alnylam Pharmaceuticals, Inc. v. Moderna, Inc., ModernaTX, Inc. & Moderna U.S., Inc., No. 22-cv-00925 (D. Del. filed July 12, 2022); and Alnylam Pharmaceuticals, Inc. v. Pfizer Inc., Pharmacia & Upjohn Co. LLC, BioNTech SE & BioNTech Manufacturing GmbH, No. 1:22-cv-00924 (D. Del. filed July 12, 2022).
Acuitas v. Arbutus and Genevant
On March 18, 2022, one day after the originally-filed Alnylam cases, LNP pioneer Acuitas sued Arbutus and Genevant in the U.S. District Court for the Southern District of New York seeking a declaratory judgment that the Pfizer Comirnaty® product does not infringe U.S. Patent Nos. 8,058,069, 8,492,359, 8,822,668, 9,364,435, 9,504,651, and 11,141,378 (all owned by Arbutus and asserted in the Moderna case) and U.S. Patent Nos. 9,006,417, 9,404,127, and 9,518,272 (all also allegedly owned by Arbutus) and that the subject Arbutus patents are invalid.
Neither Pfizer nor BioNTech have yet been named as a plaintiff in the case, but Acuitas has alleged that Pfizer and BioNTech's Comirnaty® products contain "the lipids and lipid nanoparticles innovated by Acuitas" that are grounded in certain collaborations between Acuitas and BioNTech/Pfizer. Since Acuitas has itself apparently not been accused of infringement, and Acuitas apparently does not make or sell the Comirnaty® product, one of the key early issues in this case is whether Acuitas has standing to sue.
Defendants have indicated their intention to move to dismiss for lack of standing and the court has indicated that it would entertain such a motion and invited Acuitas to amend its complaint to address arguments raised by Arbutus and Genevant in their proposed motion to dismiss. Acuitas recently (yesterday) indicated that it plans to amend its complaint by September 6, 2022. Whether that will include adding Pfizer and/or BioNTech as declaratory judgment plaintiff(s), and/or adding other facts designed to bolster Acuitas's standing argument, remains to be seen.
This case is before Judge Mary Kay Vyskocil and the caption for this case is: Acuitas Therapeutics Inc. v. Genevant Sciences GmbH & Arbutus Biopharma Corp., No. 1:22-cv-02229 (S.D.N.Y. filed Mar. 18, 2022).
CureVac v. BioNTech and Pfizer
On July 7, 2022, early pioneer and German company CureVac reportedly sued BioNTech (but not Pfizer) in the German Regional Court in Düsseldorf for infringement of one European Patent (EP1857122B1) and three German patents (DE202015009961U1, DE202021003575U1, and DE202015009974U1). These patents appear to be directed to features of the mRNA payload as well as lipids.
Within weeks of CureVac's suit in Germany, on July 25, 2022, BioNTech and Pfizer sued CureVac in the U.S. District Court for the District of Massachusetts for a declaratory judgment that they do not infringe CureVac's U.S. Patent Nos. 11,135,312, 11,149,278 and 11,241,493 (alleged U.S. counterparts to the European/German patents asserted in Germany). These are cases to watch, as BioNTech (and, by extension, Pfizer) is now confronting infringement allegations by CureVac on two continents.
The U.S. case is before Judge Nathaniel M. Gorton and the caption for that case is: BioNTech SE, BioNTech Mfg. Gmbh, and Pfizer, Inc. v. CureVac AG, No. 22-cv-11202 (D. Mass. filed July 25, 2022).
Landscape
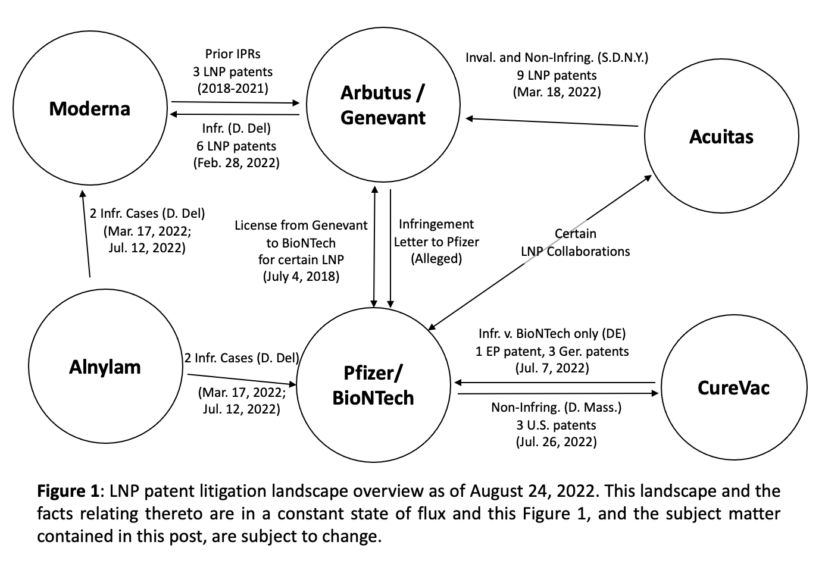
In summary, the patent litigation landscape in the mRNA space is beginning to take shape. Lipid nanoparticle pioneer Arbutus and Genevant filed the first major lawsuit by suing Moderna in February following years of conflict surrounding Arbutus's LNP technology. On another front for Arbutus and Genevant, as Acuitas alleged in its complaint, it appears that Arbutus and Genevant may have sent enforcement letters to Pfizer, perhaps signaling another future lawsuit against Pfizer and/or BioNTech.
For Acuitas's part, it is suing Arbutus and Genevant over the same patents those parties are asserting in their case against Moderna, as well as additional Arbutus patents not asserted in the Moderna case. Whether Acuitas has standing to sue remains to be determined, but in any event, the case itself could survive dismissal if Pfizer and/or BioNTech were added as a party since each may be able to establish injury in fact. The answer to this standing question could be provided as early as September 6, 2022, the date Acuitas informed the court it would be amending its complaint.
Moderna and Pfizer and BioNTech also have to contend with Alnylam, which is not currently an mRNA player, but does have patents directed to lipids utilized to deliver its small interfering RNA (SiRNA) therapeutics. It appears that the applications that led to the issuance of both of the asserted Alnylam patents were filed after at least the lipid formulation for Comirnaty® was publicly disclosed, and it should be anticipated that Alnylam could possibly seek to file for and obtain additional related patents which may also be later asserted in the Moderna and Pfizer/BioNtech cases going forward.
Finally, BioNTech and Pfizer are also entangled with CureVac, an early mRNA pioneer founded in 2000 (8 years before BioNTech and 10 years before Moderna). CureVac, too, had a COVID-19 vaccine candidate, but that therapy was not fully successful in the clinic and never reached market. CureVac's aggressive suit in Germany apparently demonstrates how seriously it feels about its infringement claims against BioNTech, and BioNTech and Pfizer must also feel strongly about their case since they are now confronting CureVac in the U.S. market by filing a declaratory judgment action against CureVac there asserting non-infringement of CureVac's corresponding U.S. patents.
All of these cases are in their very early stages, and mRNA market players should monitor the outcomes very closely. If any of the early broad LNP formulation patents are invalidated (or canceled through IPR/post-grant review (PGR) proceedings), that could allow other mRNA market players and new entrants more flexibility in utilizing a wider range of delivery formulations without having to license from such third-party patent-holders.
Alternatively, if such patent-holders overcome validity challenges during litigation, then those surviving patents would be effectively strengthened after having been battle-tested and using technology covered by those patents may require a license. As is the case with such proceedings, some patents may survive, some may not, some claims of an individual patent may survive, some may not, and all corresponding results should be monitored closely as applied to a company's pipeline.
Also, if a case settles, the subject patents are likely to survive invalidity challenges since in most settlements neither party admits or stipulates to adverse outcomes on the merits including (on the patent-holder's part) that the asserted patents are not valid. Stipulating to invalidity would likely also destroy some of the patent-holders' businesses, since it appears certain of those business models are based entirely on licensing the subject LNP technology and related know how, but not in producing and selling applicable therapeutics.
Expiration dates also play a role in the analysis, with some of the early LNP formulation patents set to expire in, for example, 2029. Given the often long go-to-market runway for pharmaceuticals and biologics, 2029 may not be that far away to stage for a commercial launch while minimizing licensing fee obligations. However, for companies already in the clinic and with a relatively shorter runway, such an expiration date is farther out and the subject patents therefore become more relevant for commercialization considerations.
In conclusion, mRNA market players should monitor this landscape and work closely with their business teams and patent counsel to set effective strategies to navigate, innovate and succeed in this complex space.
Footnote
1. For ease of reading, the individual corporate entities in this article will be referred to individually or collectively as a single corporate name without corresponding corporate suffixes (i.e., without Inc., GmbH, etc.). For example, litigating parties Arbutus Biopharma Corporation will be referred to as "Arbutus," Genevant Sciences GmbH will be referred to as "Genevant," and both Moderna Inc. and ModernaTX Inc. will be referred to collectively as "Moderna."
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.

