- with readers working within the Healthcare industries
- within Real Estate and Construction topic(s)
On Tuesday, July 30, Manatt Health hosted a webinar on how the newly enacted budget reconciliation legislation (the One Big Beautiful Bill Act or OBBBA) will impact the life sciences industry. Below are some of the key points to help you prepare. Click here to watch the full webinar.
On July 4, 2025, President Trump signed the OBBBA into law. The budget reconciliation package makes significant reforms to Medicaid and the Affordable Care Act (ACA) Marketplace. The Congressional Budget Office analysis found that the legislation would, by 2034, result in:
- More than $1 trillion cut from federal Medicaid spending
- Ten million more uninsured people in the U.S.
- A potential coverage loss to 15 million individuals stemming from both the OBBBA and the expiration of enhanced ACA subsidies at the end of this year
- Manatt's 50-state impact assessment provides additional details on a state-by-state basis
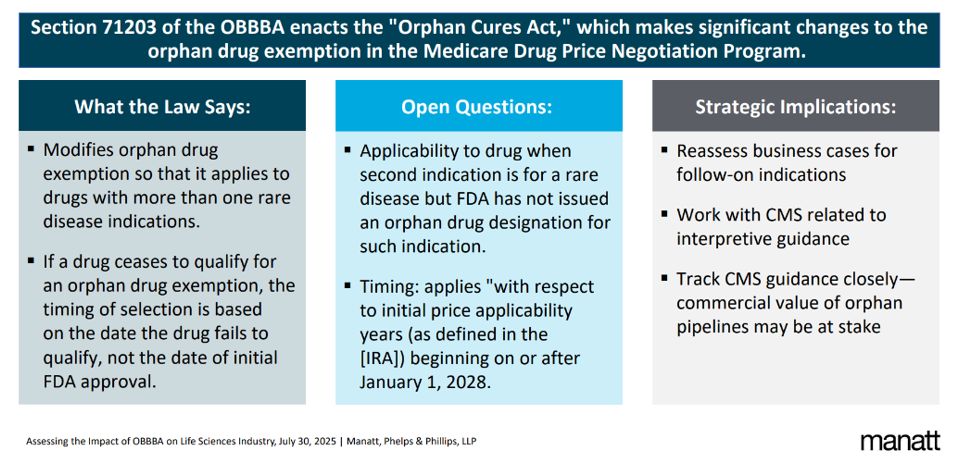
The law also expands the orphan drug exemption to the Medicare Drug Price Negotiation Program.
Those changes could have major impacts on life science companies. We've identified key areas to help you prepare.
Mitigating Coverage Losses
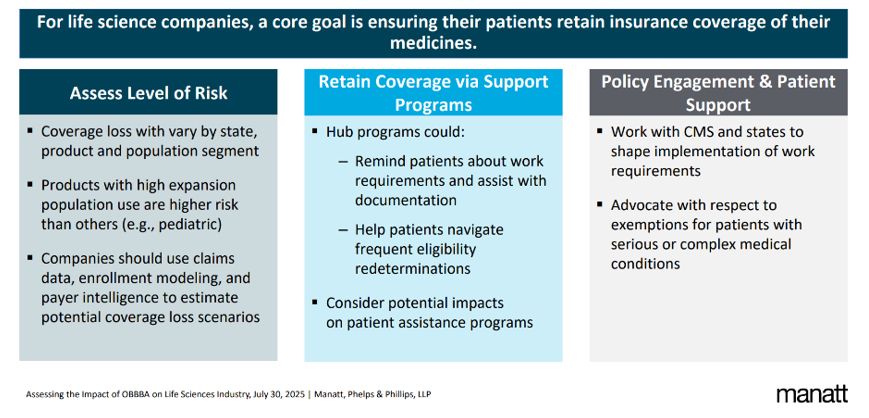
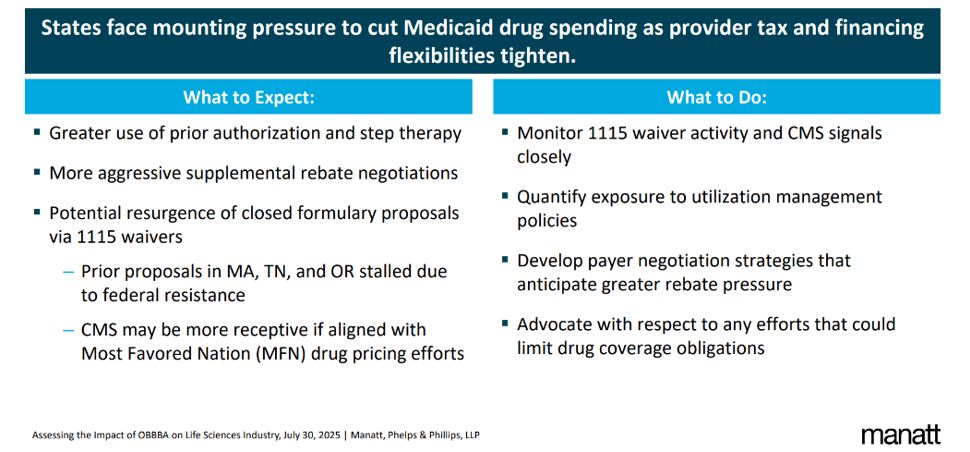
Impacts on Drug Access
Orphan Drug Protections
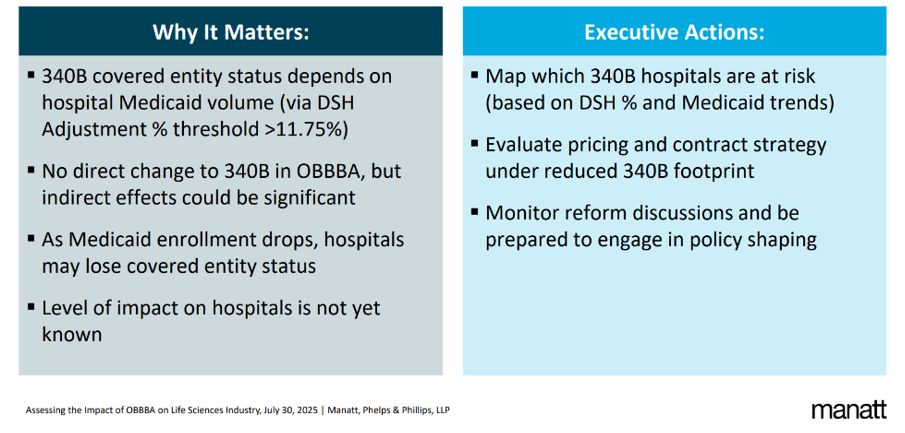
340B Covered Entity Status
Critical Factors
As life science companies prepare for the implications of the OBBBA, they will also need to consider individual state policies and the distribution of Medicaid and Marketplace coverage loss across states and populations.
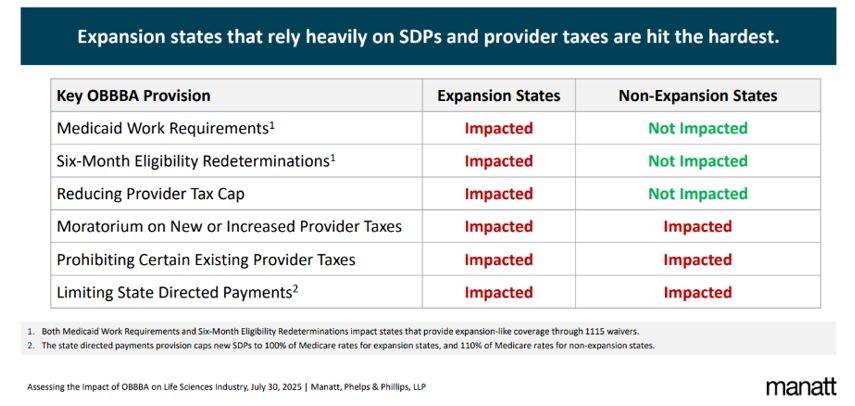
Manatt's 50-state impact assessment provides a detailed breakdown.
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.





