The purpose of patents is to drive innovation. However, many jurisdictions accept that a patent term of 20 years from filing is not always sufficient to compensate an innovator for the expense and risk of developing certain types of product. This applies particularly where regulatory approval means that a patented product cannot be sold until authorised. Countries including the USA, Australia and Japan have well established Patent Term Extension (PTE) systems to compensate for regulatory delay and, in the summer of 2021, China introduced a similar procedure.
Many countries in Europe (still including the United Kingdom despite its exit from the EU) have adopted an alternative approach to compensating for lost patent term. The purpose of restoring patents as a driver for innovation is the same but the means is rather different: rather than extend the patent itself, European countries grant an independent, sui generis, right giving up to five years of extra protection at the overlap between what has been patented and what has been authorised for sale. This new right is known as a "Supplementary Protection Certificate" (SPC) and although quite limited in scope, it can provide highly valuable protection since it comes into force at the expiry of the patent and is sharply focused on protecting the commercial product.
Since SPCs serve the same valuable purpose as PTEs but are implemented rather differently, it's worthwhile for anyone working in regulated technologies to know a little about how they are obtained and what protection they can provide. This article outlines how and when applications for SPCs are made and what protection you or your competitors might achieve from them. SPC law is complex and continues to evolve, however, so involving a knowledgeable European Patent Attorney as early as the patent drafting stage may be justified in key cases.
What products can benefit from SPC Protection?
The SPC rules allow for protection of regulated products in "Medicinal" and "Plant Protection" technologies. Medicinal products include veterinary medicines but are focused on new active ingredients. New combinations of active agents are permitted but each element of the combination must have pharmaceutical activity of its own. A combination of an active entity and an adjuvant is unlikely to succeed. Similarly, medical devices do not qualify. Although for some years it appeared that SPCs to second medical indications might be permitted, recent cases at the CJEU (Europe's highest central court) have rather squashed that hope too.
Products can only be protected by SPCs if they have a granted Marketing Authorisation effective in the relevant country. A previous SPC on a product precludes grant of a new SPC even if a new patent issues covering that product.
To be eligible for an SPC, a product must be "protected" by a "basic patent in force". The simplest requirement of this is that the relevant patent (European or National) must have granted and not have expired before you apply for your SPC. The requirement of "protection" is much less straightforward. It would be easy to assume that this means that the product must fall within the granted patent claims but sadly nothing so simple! Although falling within the scope of the claims is essential, the active agent or combination must be related to the overall invention disclosed in the patent application and each active agent must be "specifically identifiable".
On the basis of current cases, it is advisable to disclose compounds and combinations of key interest as clearly as possible, both in the claims of an application and in the description of the invention. It should be clear that the "invention" described in a patent application can be implemented with these key embodiments. Deficiencies in these disclosures cannot be rectified after filing so care at the drafting stage is essential. Given the sheer number of recent SPC cases and the continuing uncertainty in the area, patent drafts for key SPC-protectable products may benefit from review by a European Patent Attorney with up-to-date knowledge in the area.
Applying for an SPC
All SPC applications in Europe are processed by the national Patent Offices. The central patent application process at the EPO does not apply to SPCs and a separate application must be filed directly in each country of interest.
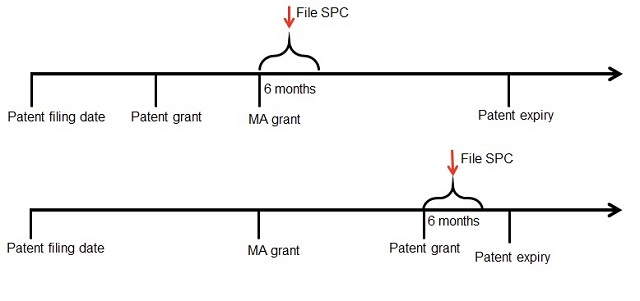
When applying for a SPC you must state both the relevant patent number and the identity of the relevant Marketing Authorisation (MA). As a result, the window for application opens as soon as both of these requirements are met – that is, once both the patent and the MA are granted. The window for application closes 6 months later (or on expiry of the patent if that's earlier). The window for filing an SPC is illustrated in the two timelines below.
If you are applying for Marketing Authorisation in Europe, it is always advisable to keep your European Patent Attorney updated on the status of your MA applications. Grant of a patent may mark the start of the SPC application window and this could be missed if your European Attorney is not aware of your MA. Similarly, if multiple patents have granted when the MA is received then they can advise on which will give best protection.
SPC Duration
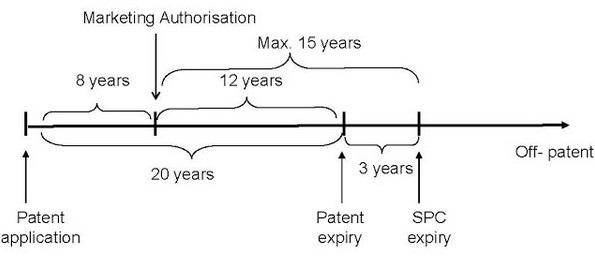
An SPC enters into force at the expiry of the relevant patent and serves to extend its protection. The aim of an SPC is to provide the holder of a Marketing Authorisation with 15 years of exclusivity after the MA is granted. However, a maximum of 5 years of additional protection is available from the SPC.
Given the above parameters, it can be seen that any patent with 10 or more years remaining when the MA is granted will receive an SPC bringing the total duration of protection up to 15 years from receipt of that MA. If the remaining patent life is less than 10 years then the target of 15 years cannot be reached but the maximum SPC duration of 5 years will be obtained. Below is shown an Example of an SPC granted with a 3-year term on a patent that has 12 years remaining after grant of the MA.
The term is calculated from the first Marketing Authorisation in any relevant European country so ensure that your representatives in Europe know of all your MA grants for the corresponding products to ensure the correct declarations are made. Although the UK is now outside the European Union, the SPC rules are incorporated into UK law and so the procedure is essentially identical in the UK, at least for the moment.
SPC Scope and Protection Conferred
The protection conferred by an SPC is the same as that provided by a granted patent, with two primary exceptions: 1) manufacture for export is not prohibited by an SPC and 2) for the final 6 months of SPC term, the product may be made and stored in preparation for local sale after SPC expiry. This is intended to encourage manufacture within Europe for export to non-protected markets.
Beyond the manufacturing waiver, the usual prohibitions on making, selling, importing etc. apply with SPCs in the same way as patents. However, protection is strictly confined to the product(s) covered by the MAs so no protection is provided, for example, for other compounds that might be claimed in the patent but not authorised for sale in the MA.
Summary
SPCs provide additional protection for regulated products in Europe where there is less than 15 years of patent term remaining after Marketing Authorisation is received. A maximum of 5 years of additional protection can be obtained.
An SPC protects products in medicinal (including veterinary) and plant protection technologies but is focused on new active agents and combinations.
A product must be covered by a MA and a patent to apply for an SPC. The patent must specifically disclose the relevant actives and their relevance to the invention.
There is a window of 6 months from grant of the patent or the MA (whichever is later) during which to apply for an SPC.
SPCs can be very valuable but many of the rules on SPCs are arcane and subject to change. Seek advice from a knowledgeable European Patent Attorney at an early stage if you think you may ultimately wish to apply for an SPC.
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.
[View Source]

