- within Law Department Performance and Real Estate and Construction topic(s)
Executive Summary
Thanks to breakthroughs in precision medicine, leading scientists have developed a growing number of cell and gene therapies (CGTs) for serious and life-threatening diseases. By addressing the root causes of disease, these transformative therapies can slow, stop, or even reverse disease progression, achieving life-changing results for patients who previously had no hope of effective treatment.
Troublingly, these breakthroughs are not accessible to all. Nationwide, the low-income people covered by the Medicaid program have reduced access to CGTs compared to those covered by Medicare or commercial insurance.1 On paper, federal law provides that all Medicaid enrollees should have timely access to Food and Drug Administration (FDA)-approved drugs and most specialty care. But in practice, patients and their caregivers can encounter barriers at each step in their journey from diagnosis to treatment.
Many of these barriers reflect certain key differences between transformative therapies and other, more familiar drugs and services. Moreover, Medicaid policies can vary significantly from state to state, and also across managed care organizations (MCOs) within a single state. In addition, although not the focus of this paper, states have identified issues concerning the high upfront cost of certain CGTs and challenges associated with managing the financial risk created by this growing class of treatments.
Transformative therapies are more like a heart transplant than a statin. These highly sophisticated therapies require specialized training to manufacture and administer. As a result, CGTs may only be available at a few "Center of Excellence" providers nationwide.
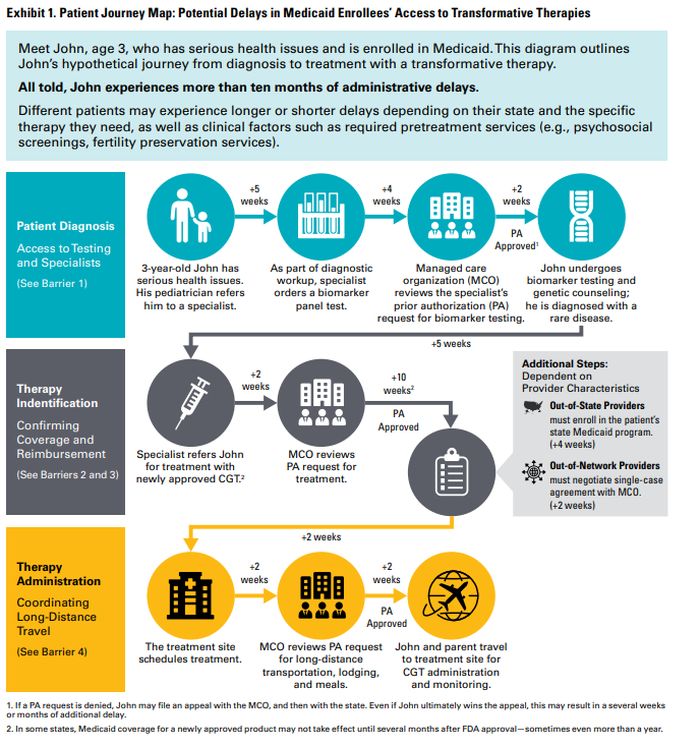
This paper identifies key barriers that can impede Medicaid patients' access to CGTs, as well as policy solutions to address those barriers at the state and federal levels. These barriers and solutions—which are summarized on the next page and presented in greater detail in the Appendices—were informed by Manatt Health's survey of relevant Medicaid policies in all 50 states and Washington, D.C., as well as a review of the literature and conversations with diverse stakeholders in the Medicaid ecosystem.
The federal government is currently implementing a Medicaid demonstration project aimed at promoting access to CGTs for Medicaid enrollees with sickle cell disease.2 A broader conversation is needed, however. The FDA has approved more than 25 CGTs to date, and that number is growing rapidly. By 2030, it's estimated that CGTs could offer life-changing results to more than 100,000 patients every year. By implementing the reforms outlined in this paper, federal and state policymakers can ensure that Medicaid enrollees are fairly represented in that number.
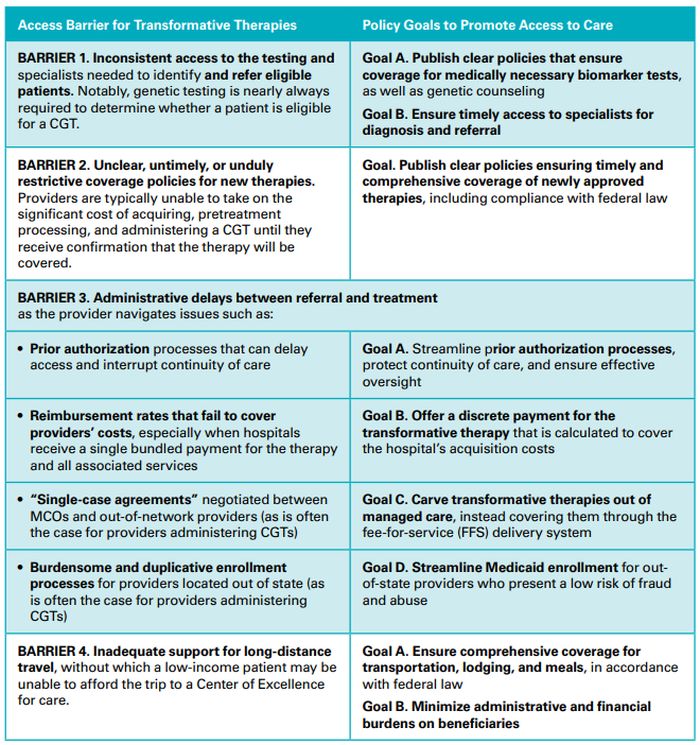
Exhibit 1. Patient Journey Map: Potential Delays in Medicaid Enrollees' Access to Transformative Therapies
Meet John, age 3, who has serious health issues and is enrolled in Medicaid. This diagram outlines John's hypothetical journey from diagnosis to treatment with a transformative therapy.
All told, John experiences more than ten months of administrative delays.
Different patients may experience longer or shorter delays depending on their state and the specific therapy they need, as well as clinical factors such as required pretreatment services (e.g., psychosocial screenings, fertility preservation services).
Background: Medicaid Enrollees Deserve Access to Transformative Therapies
Disparities in Access to Life-Changing Therapies
Being diagnosed with a serious disease can be overwhelming and life changing. However, advances in precision medicine offer new hope to patients and can make a significant difference in treatment outcomes. Over the last decade, leading scientists have developed a number of CGTs that address the root causes of genetic and chronic diseases.
CGTs go beyond alleviating the symptoms of a disease. In some cases, these therapies may cure diseases once considered uncurable, leading to recoveries for patients who were dying from treatment-resistant leukemia or suffering painfully from sickle cell anemia, for example. For other conditions, CGTs can halt or slow disease progression, preventing children from going blind due to inherited retinal diseases or helping infants with spinal muscular atrophy live longer lives free of ventilators and feeding tubes.
Troublingly, these breakthroughs are not equally accessible to all. Nationwide, the low-income people covered by the Medicaid program have reduced access to CGTs compared to those covered by Medicare or commercial insurance,3 consistent with historical trends for other novel, highly specialized therapies.4 There are also disparities within the Medicaid program: due to significant policy variations across state lines, Medicaid enrollees in some states have more reliable access than in others.5
Supporting access for Medicaid means supporting health outcomes for historically underserved groups, including:
- Low- and middle-income families. In households below 200% of the Federal Poverty Level ($62,400 for a family of four), Medicaid covers 4 out of 10 nonelderly adults and 7 out of 10 children.6
- Communities of color. As compared to non-Hispanic White people, Medicaid enrollment is 70 to 220% higher among people who are Black, Hispanic, or indigenous (i.e., American Indians, Alaska Natives, and Native Hawaiians and Pacific Islanders).7
- Rural populations. People in rural communities are more likely to be enrolled in Medicaid than those in urban centers.8
- Children with special health care needs. Nationwide, almost half of these children are covered by Medicaid across all income levels.9

Ensuring access to CGTs for Medicaid enrollees is thus a crucial component of the broader push to promote equity in precision medicine overall, as described in the sidebar.
Diagnosing the Problem: Access Barriers at Each Step in the Patient's Journey
In policy, as in medicine, identifying the right treatment depends on accurately diagnosing the problem.
On paper, federal law provides that all Medicaid enrollees should have timely access to FDA-approved drugs and most specialty care. Moreover, for children and youth under the age of 21, states must cover all medically necessary diagnostic and treatment services—including those not covered for older adults—under the comprehensive benefit for Early and Periodic Diagnostic, Screening, and Treatment (EPSDT) services.10
States vary significantly in how they implement these federal requirements, however. And within a single state, certain policies may be implemented differently by each of the state's MCOs—private plans that contract with the state to administer coverage, similar to third-party administrators for employer health plans. (See the sidebar for additional discussion of Medicaid program structure and areas of variation.)
In practice, when Medicaid-enrolled patients seek transformative therapies for rare and serious diseases, they and their caregivers can encounter barriers at each step in their journey from diagnosis to treatment, particularly in the early years following FDA approval. with respect to new transformative therapies for rare and serious diseases. Many of these barriers reflect certain key differences between transformative therapies and other, more familiar drugs and services.
CGTs are more like a heart transplant than a statin. These are highly sophisticated therapies that require specialized training to manufacture and administer. Some CGTs must be manufactured specifically for each patient. As a result, CGTs are typically available at only a few Centers of Excellence nationwide, especially in the early years following approval. These therapies address serious and life-threatening conditions that can't be managed as effectively, or at all, with maintenance medications or other, more commonly available therapies. They also typically come with higher upfront costs than many other therapies, while offering a potential cure or permanent remission.
To view the full article click here
Footnotes
1. Auletta JJ et al., Assessing Medicaid Coverage for Hematopoietic Cell Transplantation and Chimeric Antigen Receptor T Cell Therapy: A Project from the American Society for Transplantation and Cellular Therapy and the National Marrow Donor Program ACCESS Initiative. August 2023.; Beinfeld MT et al., Variation in Medicaid and commercial coverage of cell and gene therapies. October 2023.
2. Under CMS' Cell and Gene Therapy Access Model, the federal government would negotiate outcomes-based pricing agreements with CGT manufacturers, which would then be available on equal terms to states that agree to meet certain participation requirements.
3. Auletta JJ et al., Assessing Medicaid Coverage for Hematopoietic Cell Transplantation and Chimeric Antigen Receptor T Cell Therapy: A Project from the American Society for Transplantation and Cellular Therapy and the National Marrow Donor Program ACCESS Initiative. August 2023.; Beinfeld MT et al., Variation in Medicaid and commercial coverage of cell and gene therapies. October 2023.
4. Ashrafzadeh S, Asgari MM, Geller AC. The Need for Critical Examination of Disparities in Immunotherapy and Targeted Therapy Use Among Patients With Cancer. 2021.; Wong, R et al., Race/ethnicity and insurance status disparities in access to direct acting antivirals for hepatitis C virus treatment. September 2018.
5. Beinfeld MT et al., Variation in Medicaid and commercial coverage of cell and gene therapies. October 2023.; Auletta JJ et al., Assessing Medicaid Coverage for Hematopoietic Cell Transplantation and Chimeric Antigen Receptor T Cell Therapy: A Project from the American Society for Transplantation and Cellular Therapy and the National Marrow Donor Program ACCESS Initiative. November 2023.; Ballreich J et al., Coverage of genetic therapies for spinal muscular atrophy across fee-for-service Medicaid programs. December 2021.; Suma Sri Chennapragada et al., CAR-T therapy access with Louisiana Medicaid: A single institution retrospective analysis. May 2023.
6. Kaiser Family Foundation. Health Coverage & Uninsured Archives.
7. Kaiser Family Foundation. Health Coverage by Race and Ethnicity, 2010–2022. January 2024.
8. Medicaid and CHIP Payment and Access Commission. Medicaid and Rural Health. April 2021.
9. Kaiser Family Foundation. 10 Things to Know About Medicaid. June 2023.
10. Centers for Medicare & Medicaid. SHO #24-005. September 2024.
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.
[View Source]




