- within Food, Drugs, Healthcare and Life Sciences topic(s)
- with Senior Company Executives and HR
- in United States
- with readers working within the Advertising & Public Relations, Technology and Pharmaceuticals & BioTech industries
Inefficiencies in batch release processes can tie up millions in revenue, delay the production of lifesaving medicines and create compliance problems. Alvarez & Marsal (A&M) helped one 400-millioneuro German pharma company tackle these issues by implementing a tailored AI solution to digitize and analyze its batch release operations. In just three weeks, the initiative delivered transformative results including streamlined operations, improved compliance and faster time-to-market.
BACKGROUND
In the pharmaceutical industry, one of the most heavily regulated globally, managing large volumes of documentation is a universal challenge. Issues such as handling handwritten supplier records, incomplete documentation and country-specific certification requirements can lead to inefficiencies and create bottlenecks that impact both revenue and patient outcomes. For companies relying on external manufacturing, the stakes are even higher.
In this context, A&M was recently engaged by a leading pharmaceutical company to design and implement a bespoke AI-powered solution that addressed their specific needs. The client faced significant inefficiencies in their batch release process due to manual, paperheavy workflows. For instance, a team of six people in the quality department had to manually review scanned PDF documents – often hundreds of pages long and of varying quality – to identify key words and phrases necessary to confirm approval.
This repetitive process was time-consuming and prone to errors. Additional challenges included managing multilingual documentation, the need for stringent compliance checks and the lack of real-time visibility, which often led to bottlenecks and delays caused by fragmented systems, as well as increased manual workload for the quality teams.
A&M'S APPROACH: A Tailored AI Solution for Batch Release Efficiency
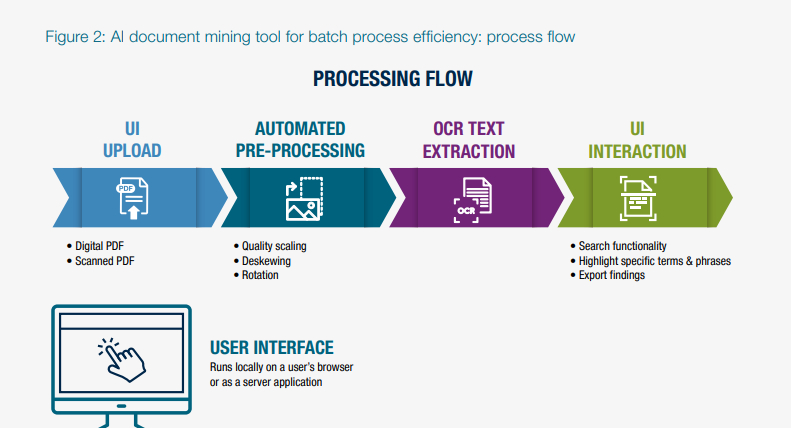
A&M's solution used Machine Learning (ML)-trained Optical Character Recognition (OCR) and Natural Language Processing (NLP) to digitize and analyze thousands of batch records and certificates of analysis. Our team tailored the implementation to align with the client's specific workflows and operational environment, ensuring it addressed their unique pain points. More specifically, the tool accurately provided location of all searched words within seconds, highlighting every occurrence in the PDF and extracting the relevant text. This reduced the batch release processing time by a significant percentage.
As part of the engagement, the client's team received extensive training and onboarding to ensure seamless adoption and effective use of the tools. While the initial scope excluded integration with systems like Laboratory Information Management System (LIMS) or Manufacturing Execution System (MES), the solution was designed to be scalable and adaptable for future enhancements.
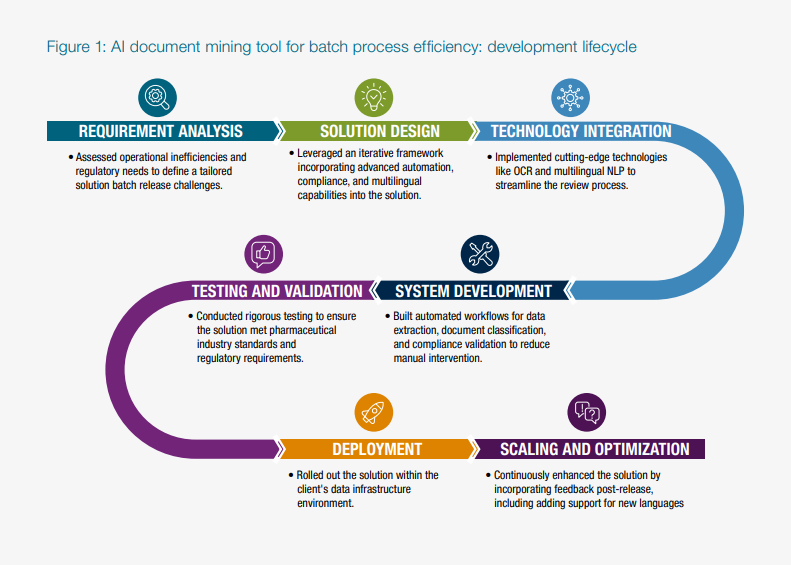
AI-POWERED TOOL FOR DOCUMENT MINING: Key Features and Benefits
The batch release process is often slowed by manual, paper-heavy workflows and fragmented systems. A&M's tailored AI solution addressed these inefficiencies through several key features:
- OCR and image pre-processing: advanced OCR and image pre-processing capabilities were implemented to ensure comprehensive information capture, even from low-quality scans or handwritten documents. This eliminated the need for manual data entry and significantly reduced errors.
- AI-driven compliance checks: automated compliance checks validated batch records and certificates of analysis against predefined regulatory requirements. The system flagged missing or incorrect data, enabling quality teams to focus on resolving exceptions rather than manually reviewing every document.
- Multilingual NLP capabilities: to address the client's multilingual needs, the tool integrated NLP capabilities to process documentation in German and English, with the flexibility to add additional languages such as French, Spanish or Chinese in the future. This feature was particularly valuable for a company operating in a globalized regulatory environment.
- Automation of repetitive tasks: Tasks such as data extraction, document classification and compliance validation were automated, reducing the manual workload on quality teams and allowing them to focus on higher-value activities like exception management.
- Dashboards and reporting: dashboards and reporting tools provided visibility into the batch release process, helping the client identify bottlenecks, track progress and ensure timely resolution of issues. Going forward, there is opportunity to productionize the dashboards for real-time insight.
- Integration with broader systems: A&M ensured the solution was future-proofed by designing it to integrate with broader systems like LIMS, MES or ERP. This enabled end-to-end process optimization and seamless data flow, further reducing manual intervention and enhancing operational efficiency in the long run.
THE RESULTS: Better Compliance, More Efficiency, Revenues Gains
Following the implementation of the tool, the client experienced significant improvements in its batch release processes and overall operational efficiency, including:
- Faster Batch Release: Reduced time-to-market for critical products, from weeks to days, preventing lost sales and meeting market demand more promptly.
- Improved Compliance: Automated checks ensured complete and accurate documentation, mitigating the risk of regulatory penalties and associated losses.
- Enhanced Operational Efficiency: Optimized resources and reduced manual errors, leading to smoother workflows and improved productivity.
- Backlog Reduction: Accelerated the clearance of backlogs of batch records, which stood at approximately two million euros in delayed revenue prior to the project starting. This initiative unlocked revenues by accelerating the release of quarantined products, and improving cash flow as a result.
Originally published 12 August 2025
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.




