From 26 May 2022, all Class A non-sterile devices (such as laboratory instruments), regardless of whether new or already on the market; CE-marked devices that do not need any involvement of notified bodies under the IVDR; new devices which do not have a notified body certificate or a declaration of conformity under the current In Vitro Diagnostic Medical Devices Directive (IVDD), must comply fully with the IVDR (see other transitional periods here).
Key Changes
The IVDR has many of the same requirements as the Regulation on Medical Devices (EU) 2017/745 (MDR), which came into full effect on 26 May 2021. However, the IVDR introduces specific system and IVD classification changes across the EU, including Ireland:
- New IVD Classification system - a new set of risk categories, from Class A (low risk) to Class D (high risk) (find our guide here)
- Conformity Assessment - Classes B, C and D IVDs will all require assessment and certification by a notified body for medical devices prior to being placed on the market. Class A IVDs are self-certified (unless sold as sterile devices).
Although the IVDR applies from 26 May 2022, the confirmation of the staggered roll out of the IVDR earlier this year was a positive step towards easing fears of an IVD certification bottleneck and product shortages (detailed here).
IVDR – Key Milestones
- 5 May 2017 – Official Journal on the EU IVDR published
- 26 May 2017 – IVDR entry into force
- 26 Nov 2017 - Earliest date notified bodies could apply for designation according to the IVDR
- 26 May 2022 – IVDR becomes fully applicable
- 26 May 2024 - Last date for placing on the market IVDs with a certificate issued under the IVDD and IVDs manufactured and used within the same health institution (in-house devices)
- 26 May 2025 - Last date for putting IVDs into service with a certificate issued under the IVDD and the transition period ends for Class D IVDs
- 26 May 2026 – Transition period ends for Class C IVDs
- 26 May 2027 – Transition period ends for Class B and Class A sterile IVDs
What are the Challenges?
Ireland has only one notified body, the National Standards Authority of Ireland (NSAI), which has yet to be designated under the IVDR. Only seven notified bodies across the EU have been designated under the IVDR to date. The NSAI expects to be designated later this year. The only option for manufacturers of IVDs in Ireland to get their product certified, is to apply to any one of the following:
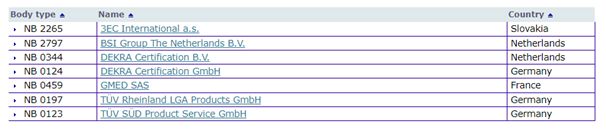
New IVD Approval Timelines
Under the IVDD, the process of approval by the NSAI took on average between three to six months.
Applications for certification to DEKRA, one of two approved
designated notified bodies in the Netherlands, currently takes an
average of one and half years. BSI (Netherlands B.V.)
has stated that it takes between nine to 11 months. The data coming
from notified bodies reveals that simple medical devices such as
test tubes will take an average of nine to 12 months to get
certified, whereas a pacemaker for example, could take one to two
years. As a result, very few products have been approved under the
IVDR to-date.
The length of time required to certify IVDs is largely dependent on
manufacturers delivering a compliant technical file (detailing the
nature, history, and data on the device) on time.
Harmonised Standards List for the IVDR – Current Reference List
Days before the application of the IVDR, the European Commission (Commission) added to the list of harmonised standards which test makers can reference to satisfy conformity requirements. The Commission added the international risk management standard EN ISO 14971:2019 to the reference list, and revised the entry for the quality management systems standard ISO 13485.
Next Steps
The advice from the NSAI and other notified bodies is that manufacturers need to be proactive and reactive under this more involved regime. Manufacturers need to engage directly with notified bodies; the earlier they do so the better. BSI, for example, is currently accepting new clients but stated that this cannot be guaranteed to continue to May 2025, when the transitional period for certain IVDs will end.
Additional national legislation is expected to be introduced in the near future by the Minister for Health to give domestic effect to certain aspects of the IVDR. We will continue to monitor further updates from the Department of Health, the Commission, the NSAI and the HPRA and keep you updated. If you require additional information on any aspect of the IVDR, please contact Charleen O'Keeffe.
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.



