- within Intellectual Property topic(s)
- in United States
- with readers working within the Law Firm industries
- within Intellectual Property, Media, Telecoms, IT, Entertainment and Strategy topic(s)
By
Vijay Pal Dalmia, Advocate
Supreme Court of India & Delhi High court
Partner: Vaish Associates Advocates
Email: vpdalmia@vaishlaw.com
Mobile: +919810081079
Patents
Background
The history of Patent law in India starts from 1911 when the Indian Patents and Designs Act, 1911 was enacted. The present Patents Act, 1970 came into force in the year 1972, amending and consolidating the existing law relating to Patents in India. The Patents Act, 1970 was again amended by the Patents (Amendment) Act, 2005, wherein product patent was extended to all fields of technology including food, drugs, chemicals and micro-organisms. After the amendment, the provisions relating to Exclusive Marketing Rights (EMRs) have been repealed, and a provision for enabling grant of compulsory license has been introduced. The provisions relating to pre-grant and post-grant opposition have been also introduced.
An invention relating to a product or a process that is new, involving inventive step and capable of industrial application can be patented in India. However, it must not fall into the category of inventions that are non-patentable as provided under sections 3 and 4 of the (Indian) Patents Act, 1970. In India, a patent application can be filed, either alone or jointly, by true and first inventor or his assignee.
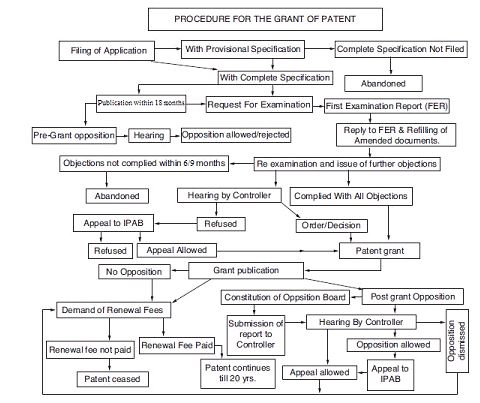
Procedure for Grant of a Patent in India
After filing the application for the grant of patent, a request for examination is required to be made for examination of the application in the Indian Patent Office within 48 months from the date of priority of the application or from the date of filing of the application. After the first examination report is issued, the applicant is given an opportunity to meet the objections raised in the report. The applicant has to comply with the requirements within 6 months from the issuance of the first examination report which may be extended for further 3 months on the request of the applicant. If the requirements of the first examination report are not complied with within the prescribed period of 9 ;months, then the application is treated to have been abandoned by the applicant. After the removal of objections and compliance of requirements, the patent is granted and notified in the Patent Office Journal. The process of the grant of patent in India can also be understood from the following flow chart:
Filing of Application for Grant of Patent in India by Foreigners
India being a signatory to the Paris Convention for the Protection of Industrial Property, 1883 and the Patent Cooperation Treaty (PCT), 1970, a foreign entity can adopt any of the aforesaid treaties for filing of application for grant of patent in India.
Where an application for grant of patent in respect of an invention in a Convention Country has been filed, then similar application can also be filed in India for grant of patent by such applicant or the legal representative or assignee of such person within 12 months from the date on which the basic application was made in the Convention Country, ie, the home country. The priority date in such a case is considered as the date of making of the basic application.
Pre-grant Opposition
A representation for pre-grant opposition can be filed by any person under s 11A of the Patents Act, 1970 within six months from the date of publication of the application, as amended (the "Patents Act") or before the grant of patent. The grounds on which the representation can be filed are provided under section 25(1) of the Patents Act. There is no fee for filing representation for pre-grant opposition. Representation for pre-grant opposition can be filed even though no request for examination has been filed. However, the representation will be considered only when a request for examination is received within the prescribed period.
Post-grant Opposition
Any interested person can file post-grant opposition within twelve months from the date of publication of the grant of patent in the official journal of the patent office.
Grounds for Opposition
Some of the grounds for filing pre-and post-grant opposition are as under:
(a) Patent wrongfully obtained;
(b) Prior publication;
(c) The invention was publicly known or publicly used in India before the priority date of that claim;
(d) The invention is obvious and does not involve any inventive step;
(e) That the subject of any claim is not an invention within the meaning of this Act, or is not patentable under this Act;
(f) Insufficient disclosure of the invention or the method by which it is to be performed;
(g) That in the case of a patent granted on convention application, the application for patent was not made within twelve months from the date of the first application for protection for the invention made in a convention country or in India;
(h) That the complete specification does not disclose or wrongly mentions the source and geographical origin of biological material used for the invention; and
(i) That the invention was anticipated having regard to the knowledge, oral or otherwise, available within any local or indigenous community in India or elsewhere.
Term of Patent
The term of every patent in India is 20 years from the date of filing the patent application, irrespective of whether it is filed with provisional or complete specification. However, in case of applications filed under the Patent Cooperative Treaty (PCT), the term of 20 years begins from the international filing date.
Payment of Renewal Fee
It is important to note that a patentee has to renew the patent every year by paying the renewal fee, which can be paid every year or in lump sum.
Restoration of Patent
A request for restoration of patent can be filed within eighteen months from the date of cessation of patent along with the prescribed fee. After the receipt of the request, the matter is notified in the official journal for further processing of the request.
Patent of Biological Material
If the invention uses a biological material which is new, it is essential to deposit the same in the International Depository Authority (IDA) prior to the filing of the application in India in order to supplement the description. If such biological materials are already known, in such a case it is not essential to deposit the same. The IDA in India located at Chandigarh is known as Institute of Microbial Technology (IMTECH).
Rights granted by a Patent
If the grant of the patent is for a product, then the patentee has a right to prevent others from making, using, offering for sale, selling or importing the patented product in India. If the patent is for a process, then the patentee has the right to prevent others from using the process, using the product directly obtained by the process, offering for sale, selling or importing the product in India directly obtained by the process.
Before filing an application for grant of patent in India, it is important to note "What is not Patentable in India?" Any invention which is (a) frivolous, (b) obvious, (c) contrary to well established natural laws, (d) contrary to law, (e) morality, (f) injurious to public health, (g) a mere discovery of a scientific principle, (h) the formulation of an abstract theory, (i) a mere discovery of any new property or new use for a known substance or process, machine or apparatus, (j) a substance obtained by a mere admixture resulting only in the aggregation of the properties of the components thereof or a process for producing such substance, (k) a mere arrangement or rearrangement or duplication of known devices, (l) a method of agriculture or horticulture and (m) inventions relating to atomic energy, are not patentable in India.
Maintainability of Secrecy by the Indian Patent Office (IPO)
All patent applications are kept secret up to eighteen months from the date of filing or priority date, whichever is earlier, and thereafter they are published in the Official Journal of the Patent Office published every week. After such publication of the patent application, public can inspect the documents and may take the photocopy thereof on the payment of the prescribed fee.
Compulsory Licensing
One of the most important aspects of Indian Patents Act, 1970, is compulsory licensing of the patent subject to the fulfillment of certain conditions. At any time after the expiration of three years from the date of the sealing of a patent, any person interested may make an application to the Controller of Patents for grant of compulsory license of the patent, subject to the fulfillment of following conditions, ie,
- the reasonable requirements of the public with respect to the patented invention have not been satisfied;
- that the patented invention is not available to the public at a reasonable price; or
- that the patented invention is not worked in the territory of India.
It is further important to note that an application for compulsory licensing may be made by any person notwithstanding that he is already the holder of a licence under the patent.
For the purpose of compulsory licensing, no person can be stopped from alleging that the reasonable requirements of the public with respect to the patented invention are not satisfied or that the patented invention is not available to the public at a reasonable price by reason of any admission made by him, whether in such a licence or by reason of his having accepted such a licence.
The Controller, if satisfied that the reasonable requirements of the public with respect to the patented invention have not been satisfied or that the patented invention is not available to the public at a reasonable price, may order the patentee to grant a licence upon such terms as he may deem fit. However, before the grant of a compulsory license, the Controller of Patents shall take into account following factors:
- The nature of invention;
- The time elapsed, since the sealing of the patent;
- The measures already taken by the patentee or the licensee to make full use of the invention;
- The ability of the applicant to work the invention to the public advantage;
- The capacity of the applicant to undertake the risk in providing capital and working the invention, if the application for compulsory license is granted;
- As to the fact whether the applicant has made efforts to obtain a license from the patentee on reasonable terms and conditions;
- National emergency or other circumstances of extreme urgency;
- Public non-commercial use; and
- Establishment of a ground of anti-competitive practices adopted by the patentee.
The grant of compulsory license cannot be claimed as a matter of right, as the same is subject to the fulfilment of above conditions and discretion of the Controller of Patents. Further judicial recourse is available against any arbitrary or illegal order of the Controller of Patents for grant of compulsory license. In 2012, the Controller of Patents has granted the first compulsory license to Natco Pharma to sell a generic version of the patented cancer drug 'Nexavar' in India. However, the subsequent applications, by BDR Pharmaceuticals for Bristol-Myers Squibb's Dasatinib and Lee Pharma for AstraZeneca's Saxagliptin, to sell generic version of these patented drugs were rejected.
The Government of India has while exercising its power under section 66 of the Patents Act, 1970 in the Public Interest, revoked Patent No. 252093, entitled "a synergistic, ayurvedic functional food bioactive composition (Cinata) and a process of preparation thereof" granted to m/s Avesthagen Ltd., on the ground that the aforesaid patent is prejudicial to public.
Infringement of Patent
Patent infringement proceedings can only be initiated after grant of patent in India but may include a claim retrospectively from the date of publication of the application for grant of the patent. Infringement of a patent consists of the unauthorised making, importing, using, offering for sale or selling any patented invention within the India. Under the (Indian) Patents Act, 1970 only a civil action can be initiated in a Court of Law. Further, a suit for infringement can be defended on various grounds including the grounds on which a patent cannot be granted in India and based on such defence, revocation of Patent can also be claimed.
Licensing and Assignment of Patent
An assignment in a patent or a share in a patent or a mortgage, license or the creation of any other interest in a patent is permissible. In the case of patents, assignment is valid only when it is in writing and the agreement is reduced to the form of a document embodying all the terms and conditions governing the rights and obligations of the parties to the agreement. The application for registration is required to be made by the transferee in the prescribed form.
© 2017, Vaish Associates Advocates,
All rights reserved
Advocates, 1st & 11th Floors, Mohan Dev Building 13, Tolstoy
Marg New Delhi-110001 (India).
The content of this article is intended to provide a general guide to the subject matter. Specialist professional advice should be sought about your specific circumstances. The views expressed in this article are solely of the authors of this article.


