- FDA and EMA both approve additional biosimilar versions of Humira® (adalimumab).
- FDA also approves its third biosimilar version of Neupogen® (filgrastim).
- EMA has not approved any new biosimilars in 2022, but has recommended approval of teriparatide biosimilar Sondelbay and the pegfilgrastim biosimilar Stimufend.
As pharmaceutical drug costs attract increasing media attention and political scrutiny, a growing number of biosimilar drugs are set to enter the U.S. and European markets in the coming years. Global sales for the top ten branded biologic drugs totaled approximately $85 billion in 20201. In a September 2020 report, the IQVIA Institute for Human Data Science estimated biosimilar sales totaling $80 billion over the next five years compared to $14 billion during the previous five years (2015-2019), and that the availability and use of biosimilar medicines would reduce U.S. drug costs by $100 billion through 2024. In a January 2022 report, IQVIA updated global estimates showing estimated biosimilar sales of about $40 billion in 2025 and $75 billion in 2030.
In the FDA's Center for Drug Evaluation and Research's (CDER) annual report, the FDA highlighted the three biosimilar approvals in 2020 under the Biologics Price Competition and Innovation Act (BPCIA) of 2009, which was "designed to create competition, increase patient access, and potentially reduce cost of important therapies." The FDA's Biosimilars Action Plan, unveiled in 2018, has been designed to aid the development of a market for biosimilars in order to increase competition for biologic drugs, which make up 40% of U.S. pharmaceutical spending. Competition in the heavily regulated marketplace for these blockbuster therapeutics is expected to substantially impact the pharmaceutical industry and national health systems. To date, the U.S. has considerably lagged behind Europe's expansion of biosimilar drug options.
Since 2005, the biosimilar regulatory framework in Europe has been implemented through the Committee for Medicinal Products for Human Use (CHMP) under the European Medicines Agency (EMA). The CHMP provides initial assessments for marketing authorization of new medicines that are ultimately approved centrally by the EMA. Since Sandoz's somatotropin biosimilar, Omnitrope®, was first authorized on April 12, 2006, an additional 83 applications have been approved in Europe. Fourteen of the authorizations have been withdrawn post-approval (Table 1).
The U.S. did not implement a regulatory framework for biosimilar evaluation until after enactment of the Biologics Price Competition and Innovation Act (BPCIA) of 2009. Given that the first U.S. biosimilar drug was approved almost a decade after the first in Europe, the number of authorized biosimilar drugs in Europe far exceeds the number of biosimilars approved in the United States. Sandoz's filgrastim biosimilar, Zarxio®, received the first U.S. approval in 2015, whereas nine filgrastim biosimilars have been approved in Europe dating back to multiple authorizations in 2008. Zarxio® (in the U.S.) and Zarzio® (in Europe) are biosimilar to the reference product Neupogen® marketed by Amgen and originally licensed in 1991. Subsequent to Zarxio®'s approval, 33 other biosimilar drugs have gained U.S. approval to date including two interchangeable products (Table 2)
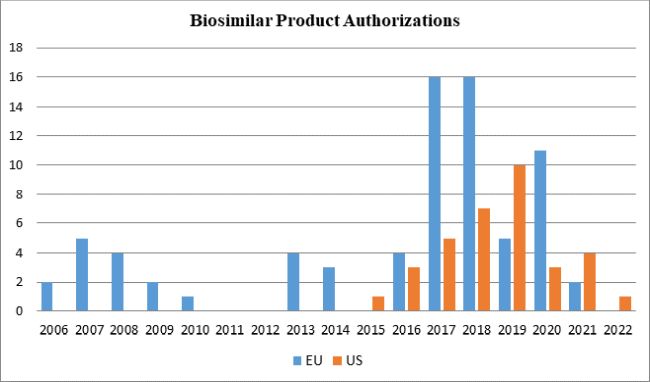
As illustrated in the following graph, while the EU's significant head start led to an imbalance in the number of biosimilar drugs available in the respective markets, the EU's relatively higher rate of approvals in recent years has widened its lead over the United States, although the U.S. FDA reversed that trend in 2019 with ten approvals. Through 2021 and thus far in 2022, relatively fewer biosimilars have been approved by both FDA and EMA than in prior years. Given the increasing competition between biosimilar manufacturers in Europe, four EMA-authorized biosimilar products were withdrawn in 2021.
A recent study of U.S. biosimilar approvals found that most comparative efficacy trials conducted to obtain FDA approval for a biosimilar had a tendency to be larger, longer, and more costly than clinical trials required for originator products. Moreover, the FDA requires animal studies whereas the EMA does not require animal studies to approve a biologic product. Further, given the difficult patent litigation and competitive landscapes, there appear to be fewer biosimilar BLAs than in 2017-2019, and launches of FDA-approved adalimumab and rituximab biosimilars are delayed due to settlements of patent litigations. Thus, in addition to the patent litigation landscape, there are regulatory hurdles and costs faced by biosimilar applicants that deter or delay biosimilar products from reaching the U.S. market.
Currently, fourteen biosimilar applications are under review by the EMA for marketing authorization (Table 3). As an increasing number of patents expire on blockbuster biologic drugs, the number of abbreviated biologics license applications is also increasing. Biosimilars for more than 28 different original biologics are currently navigating biosimilar pathways or are in late stage development in the U.S. (Table 4).
On December 20, 2021, the FDA approved Coherus' adalimumab YusimryTM biosimilar. "YUSIMRY represents an enormous commercial opportunity for Coherus as we continue our mission of increasing patient access to important biologic medicines while at the same time lowering the cost of care," said Paul Reider, Chief Commercial Officer of Coherus. "Humira is the top-selling drug in the U.S. with 2020 net sales exceeding $16 billion, and demand is high across the healthcare ecosystem for a less expensive Humira biosimilar. We will deliver a compelling value proposition to all stakeholders and look forward to launching YUSIMRY in 2023." On February 28, 2022, the FDA approved Amneal and Kashiv's filgrastim ReleukoTM biosimilar. "The U.S. approval of our first biosimilar is a very significant milestone for Amneal. Biosimilars represent the next wave of providing access to affordable medicines in the U.S. We are building a global biosimilars business by leveraging partner assets to start and then leveraging our own key capabilities over time. Our goal is to become a meaningful long-term player in biosimilars," said Chirag and Chintu Patel, Co-Chief Executive Officers.
Table 1. European Medicines Agency List of Approved Biosimilar Drugs (updated March 13, 2022).
| Biosimilar Proprietary Name |
Drug Product |
Owner | Status? | Authorization Date |
| Abasaglar (previously Abasria) |
Insulin Glargine | Eli Lilly Regional Operations GmbH |
Authorized | 9/9/2014 |
| Abevmy | Bevacizumab | Mylan IRE Healthcare Limited | Authorized | 4/21/2021 |
| Abseamed | Epoetin Alfa | Medice Arzneimittel Pütter GmbH & Co. Kg | Authorized | 8/28/2007 |
| Accofil | Filgrastim | Accord Healthcare Ltd | Authorized | 9/18/2014 |
| Admelog | Insulin lispro | Sanofi | Authorized | 5/19/2017 |
| Alpheon | Recombinant Human Interferon Alfa-2a |
Biopartners GmbH | Refused | – |
| Alymsys | Bevacizumab | Mabxience Research SL | Authorized | 3/26/2021 |
| Amgevita | Adalimumab | Amgen Europe | Authorized | 3/22/2017 |
| Amsparity | Adalimumab | Pfizer Europe MA EEIG | Authorized | 2/13/2020 |
| Aybintio | Bevacizumab | Samsung Bioepis NL B.V. | Authorized | 8/20/2020 |
| Bemfola | Follitropin Alfa | Gedeon Richter Plc. | Authorized | 3/27/2014 |
| Benepali | Etanercept | Samsung Bioepis Uk Limited (Sbuk) |
Authorized | 1/14/2016 |
| Binocrit | Epoetin Alfa | Sandoz GmbH | Authorized | 8/28/2007 |
| Biograstim | Filgrastim | Abz-Pharma GmbH | Withdrawn | 9/15/2008 |
| Blitzima | Rituximab | Celltrion | Authorized | 7/13/2017 |
| Byooviz | Ranibizumab | Samsung Bioepis | Authorized | 8/18/2021 |
| Cyltezo | Adalimumab | Boehringer Ingelheim International GmbH |
Authorized Withdrawn |
11/10/2017 1/15/2019 |
| Epoetin Alfa Hexal | Epoetin Alfa | Hexal Ag | Authorized | 8/28/2007 |
| Equidacent | Bevacizumab | Centus Biotherapeutics Europe Limited |
Authorized Withdrawn |
9/25/2020 10/11/2021 |
| Erelzi | Etanercept | Sandoz GmbH | Authorized | 6/23/2017 |
| Filgrastim Hexal | Filgrastim | Hexal Ag | Authorized | 6/2/2009 |
| Filgrastim ratiopharm | Filgrastim | Ratiopharm GmbH | Withdrawn | 9/15/2008 |
| Flixabi | Infliximab | Samsung Bioepis Uk Limited (SBUK) |
Authorized | 5/26/2016 |
| Fulphila | Pegfilgrastim | Mylan S.A.S. | Authorized | 11/20/2018 |
| Grastofil | Filgrastim | Apotex Europe Bv | Authorized | 10/18/2013 |
| Grasustek | Pegfilgrastim | Juta Pharma GmbH | Authorized | 4/26/2019 |
| Halimatoz | Adalimumab | Sandoz GmbH |
Authorized Withdrawn |
7/26/2018 12/18/2020 |
| Hefiya | Adalimumab | Sandoz GmbH | Authorized | 7/26/2018 |
| Herzuma | Trastuzumab | Celltrion Healthcare Hungary Kft. | Authorized | 2/9/2018 |
| Hukyndra | Adalimumab | Stada Arzneimittel AG | Authorized | 11/15/2021 |
| Hulio | Adalimumab | Mylan S.A.S. | Authorized | 9/19/2018 |
| Hyrimoz | Adalimumab | Sandoz GmbH | Authorized | 7/26/2018 |
| Idacio | Adalimumab | Fresenius Kabi Deutschland GmbH | Authorized | 4/2/2019 |
| Imraldi | Adalimumab | Samsung Bioepis UK Limited (SBUK) | Authorized | 8/24/2017 |
| Inflectra | Infliximab | Hospira Uk Limited | Authorized | 9/10/2013 |
| Inhixa | Enoxaparin Sodium | Techdow Europe Ab | Authorized | 9/15/2016 |
| Insulin aspart Sanofi | Insulin aspartate | Sanofi-Aventis groupe | Authorized | 7/26/2020 |
| Kanjinti | Trastuzumab | Amgen/Allergan | Authorized | 5/16/2018 |
| Kirsty (previously Kixelle) | Insulin aspart | Mylan | Authorized | 2/8/2021 |
| Kromeya | Adalimumab | Fresenius Kabi Deutschland GmbH |
Authorized Withdrawn |
4/2/2019 12/17/2019 |
| Lextemy | Bevacizumab | Mylan IRE Healthcare Limited |
Authorized Withdrawn |
4/21/2021 6/21/2021 |
| Libmyris | Adalimumab | Stada Arzneimittel AG | Authorized | 11/12/2021 |
| Livogiva | Teriparatide | Theramex Ireland Limited | Authorized | 8/27/2020 |
| Lusduna | Insulin Glargine | Merck Sharp & Dohme Limited |
Authorized Withdrawn |
4/1/2017 10/29/2018 |
| Lyumjev | Insulin lispro | Eli Lilly Nederland B.V. | Authorized | 3/24/2020 |
| Movymia | Teriparatide | Stada Arzneimittel Ag | Authorized | 1/11/2017 |
| Mvasi | Bevacizumab | Amgen Europe B.V. | Authorized | 1/15/2018 |
| Nepexto | Etanercept | Mylan and Lupin | Authorized | 6/4/2020 |
| Nivestim | Filgrastim | Hospira Uk Ltd | Authorized | 6/8/2010 |
| Nyvepria | Pegfilgrastim | Pfizer Europe MA EEIG | Authorized | 11/19/2020 |
| Ogivri | Trastuzumab | Viatris | Authorized | 12/12/2018 |
| Omnitrope | Somatropin | Sandoz GmbH | Authorized | 4/12/2006 |
| Onbevzi | Bevacizumab | Samsung Bioepis Co., Ltd. | Authorized | 1/13/2021 |
| Ontruzant | Trastuzumab | Samsung Bioepis Co., Ltd. | Authorized | 11/17/2017 |
| Ovaleap | Follitropin Alfa | Teva Pharma B.V. | Authorized | 9/27/2013 |
| Oyavas | Bevacizumab | STADA Arzneimittel AG | Authorized | 3/26/2021 |
| Pegfilgrastim Mundipharma (Cegfila) | Pegfilgrastim | Mundipharma Biologics S.L. | Authorized | 12/19/2019 |
| Pelgraz | Pegfilgrastim | Accord Healthcare Limited | Authorized | 9/25/2018 |
| Pelmeg | Pegfilgrastim | Cinfa Biotech S.L. | Authorized | 11/20/2018 |
| Qutavina | Teriparatide | EuroGenerics Holdings BV |
Authorized Withdrawn |
8/31/2020 11/26/2020 |
| Ratiograstim | Filgrastim | Ratiopharm GmbH | Authorized | 9/15/2008 |
| Remsima | Infliximab | Celltrion Healthcare Hungary Kft. |
Authorized | 9/10/2013 |
| Retacrit | Epoetin Zeta | Hospira Uk Limited | Authorized | 12/18/2007 |
| Ritemvia | Rituximab | Celltrion |
Authorized Withdrawn |
7/13/2017 6/21/2021 |
| Rituzena (previously Tuxella) | Rituximab | Celltrion |
Authorized Withdrawn |
7/13/2017 April 12, 2019 |
| Rixathon | Rituximab | Sandoz GmbH | Authorized | 6/15/2017 |
| Riximyo | Rituximab | Sandoz GmbH | Authorized | 6/15/2017 |
| Ruxience | Rituximab | Pfizer Europe MA EEIG | Authorized | 4/1/2020 |
| Semglee | Insulin glargine | Mylan S.A.S. | Authorized | 3/27/2018 |
| Silapo | Epoetin Zeta | Stada Arzneimittel Ag | Authorized | 12/18/2007 |
| Solumarv | Insulin Human | Marvel Lifesciences Ltd | Refused | – |
| Solymbic | Adalimumab | Amgen Europe |
Authorized Withdrawn |
3/22/2017 6/15/2018 |
| Terrosa | Teriparatide | Gedeon Richter Plc. | Authorized | 1/4/2017 |
| Tevagrastim | Filgrastim | Teva GmbH | Authorized | 9/15/2008 |
| Thorinane | Enoxaparin Sodium | Pharmathen S.A. | Authorized | 9/15/2016 |
| Trazimera | Trastuzumab | Pfizer | Authorized | 7/26/2018 |
| Truxima | Rituximab | Celltrion Healthcare Hungary Kft. |
Authorized | 2/17/2017 |
| Udenyca | Pegfilgrastim | Coherus/ERA Consulting GmbH |
Authorized Withdrawn |
9/25/2018 2/4/2021 |
| Yuflyma | Adalimumab | Celltrion Healthcare Hungary Kft. |
Authorized | 2/11/2021 |
| Valtropin | Somatropin | Biopartners GmbH | Withdrawn | 4/24/2006 |
| Zarzio | Filgrastim | Sandoz GmbH | Authorized | 2/6/2009 |
| Zercepac | Trastuzumab | Accord Healthcare S.L.U. | Authorized | 7/28/2020 |
| Zessly | Infliximab | Sandoz GmbH | Authorized | 5/18/2018 |
| Ziextenzo | Pegfilgrastim | Sandoz GmbH | Authorized | 11/22/2018 |
| Zirabev | Bevacizumab | Pfizer | Authorized | 2/14/2019 |
Table 2. U.S. Food and Drug Administration List of Approved Biosimilar Drugs.
| No. | Drug Product | Company | Reference Product and Sponsor | Marketing Status | FDA Approval Date |
| 34 |
Releuko (filgrastim-ayow) |
Kashiv Biosciences & Amneal Pharmaceuticals |
Amgen Neupogen® |
Not Available | 2/28/2022 |
| 33 | YusimryTM (adalimumab-aqvh) | Coherus |
AbbVie Humira® |
Not Available
|
12/20/2021 |
| 32 |
RezvoglarTM (insulin glargine-aglr) |
Eli Lilly |
Sanofi Lantus® |
Not Available
|
12/20/2021 |
| 31 |
ByoovizTM (ranibizumab-nuna) |
Samsung Bioepis and Biogen |
Genentech Lucentis® |
Not Available Launch Delayed to June 2022 |
9/17/2021 |
| 30 |
SemgleeTM (insulin glargine-yfqn) INTERCHANGEABLE |
Viatris and Biocon Biologics |
Sanofi Lantus® |
Launched November 2021 |
7/28/2021 |
| 29 |
RiabniTM (rituximab-arrx) |
Amgen | Biogen and Genentech Rituxan® |
Launched January 2021 |
12/17/2020 |
| 28 |
HulioTM (adalimumab-fkjp) |
Mylan |
AbbVie Humira® |
Not available Launch Delayed to 2023 |
7/6/2020 |
| 27 | NyvepriaTM (pegfilgrastim-apgf) | Pfizer |
Amgen Neulasta® |
Launched January 2021 |
6/10/2020 |
| 26 |
AvsolaTM (infliximab-axxq) |
Amgen |
Janssen Remicade® |
Launched July 2020 |
12/6/2019 |
| 25 |
AbriladaTM (adalimumab-afzb) |
Pfizer |
AbbVie Humira® |
Not available Launch Delayed to 2023 |
11/15/2019 |
| 24 |
ZiextenzoTM (pegfilgrastim-bmez) |
Sandoz |
Amgen Neulasta® |
Launched November 2019 | 11/4/2019 |
| 23 | HadlimaTM (adalimumab-bwwd) | Samsung Bioepis |
AbbVie Humira® |
Not available Launch Delayed to 2023 |
7/23/2019 |
| 22 | RuxienceTM (rituximab-pvvr) | Pfizer | Biogen and Genentech Rituxan® | Launched January 2020 | 7/23/2019 |
| 21 | ZirabevTM (bevacizumab-bvzr) | Pfizer |
Genentech/Roche Avastin® |
Launched December 2019 | 6/28/2019 |
| 20 | KanjintiTM (trastuzumab-anns) | Amgen |
Roche/Genentech Herceptin® |
Launched July 2019 |
6/13/2019 |
| 19 |
EticovoTM (etanercept-ykro) |
Samsung Bioepis |
Amgen Enbrel® |
Not available | 4/25/2019 |
| 18 | TrazimeraTM (trastuzumab-qyyp) | Pfizer |
Roche/Genentech Herceptin® |
Launched February 2020 | 3/11/2019 |
| 17 | OntruzantT (trastuzumab-dttb) | Samsung Bioepis |
Roche/Genentech Herceptin® |
Launched April 2020 |
1/18/2019 |
| 16 | HerzumaT (trastuzumab-pkrb) | Celltrion and Teva |
Roche/Genentech Herceptin® |
Launched March 2020 |
12/14/2018 |
| 15 | TruximaT (rituximab-abbs) | Celltrion and Teva | Biogen and Genentech Rituxan® | Launched November 2019 | 11/28/2018 |
| 14 | UdenycaT (pegfilgrastim-cbqv) | Coherus BioSciences |
Amgen Neulasta® |
Launched January 2019 | 11/2/2018 |
| 13 | HyrimozT (adalimumab-adaz) | Sandoz |
AbbVie Humira® |
Not available Launch Delayed to 2023 |
10/30/2018 |
| 12 |
NivestymTM (filgrastim-aafi) |
Pfizer |
Amgen Neupogen® |
Launched October 2018 | 7/20/2018 |
| 11 | FulphilaTM (pegfilgrastim-jmdb) | Mylan/Biocon |
Amgen Neulasta® |
Launched July 2018 | 6/4/2018 |
| 10 |
Retacrit® (epoetin alfa-epbx) |
Pfizer |
Janssen Procrit® |
Launched November 2018 | 5/15/2018 |
| 9 |
Ixifi® (infliximab-qbtx) |
Pfizer |
Janssen Remicade® |
Not Available | 12/13/2017 |
| 8 |
Ogivri® (trastuzumab-dkst) |
Mylan/Biocon |
Roche/Genentech Herceptin® |
Launched December 2019 |
12/01/2017 |
| 7 | MvasiTM (bevacizumab-awwb) |
Amgen Allergan |
Genentech/Roche Avastin® |
Launched July 2019 |
9/14/2017 |
| 6 |
CyltezoTM (adalimumab-adbm) INTERCHANGEABLE |
Boehringer Ingelheim International GmbH |
AbbVie Humira® |
Not available Launch Delayed to July 2023 |
8/25/2017 |
| 5 |
Renflexis® (infliximab-abda) |
Samsung Bioepis |
Janssen Remicade® |
Launched July 2017 | 4/21/2017 |
| 4 | Amjevita® (adalimumab-atto) | Amgen |
AbbVie Humira® |
Not available Launch Delayed to Jan. 31, 2023 |
9/23/2016 |
| 3 |
Erelzi ® (etanercept-szzs) |
Sandoz |
Amgen Enbrel® |
Not Available | 8/30/2016 |
| 2 |
Inflectra® (infliximab-dyyb) |
Celltrion/Pfizer |
Janssen Remicade® |
Launched Nov. 2016 | 4/05/2016 |
| 1 |
Zarxio® (filgrastim-sndz) |
Sandoz |
Amgen Neupogen® |
Launched Sept. 2015 |
03/06/2015 |
Table 3. European Medicines Agency List of Biosimilars Under Evaluation for Marketing Approval (Source: EMA list of applications for new human medicines compiled on March 8, 2022 and published on March 11, 2022).
| Drug Product | Reference Product Proprietary Name | Reference Product Sponsor | Number of Applications |
| Bevacizumab | Avastin® | Genentech/Roche | 2 |
| Eptacog alfa | NovoSeven® | Novo Nordisk | 1 |
| Filgrastim | 1 | ||
| Pegfilgrastim | Neulasta® | Amgen | 2 |
| Ranibizumab | Lucentis® | Genentech | 3 |
| Teriparatide | Forteo®/Forsteo® | Eli Lilly | 2 |
| Trastuzumab | Herceptin® | Roche/Genentech | 3 |
Table 4. Biologics having already expired or nearing primary patent expiry in the U.S. and biologics that have biosimilars in the regulatory pipeline.
| Drug Product |
Primary U.S. Patent Expiry |
| OnabotulinumtoxinA (Botox®) | Primary patents long-expired, various use patents pending |
| Insulin products (various) | Primary patents long-expired |
| Filgrastim (Neupogen®) | 2013 |
| Epoetin alfa (Epogen®) | 2013 |
| Pegfilgrastim (Neulasta®) | 2015 |
| Adalimumab (Humira®) | 2016 |
| Rituximab (Rituxan®) | 2018 |
| Cetuximab (Erbitux®) | 2018 |
| Omalizumab (Xolair®) | 2018 |
| Infliximab (Remicade®) | 2018 |
| Teriparatide (Forteo®) | 2019 |
| Bevacizumab (Avastin®) | 2019 |
| Trastuzumab (Herceptin®) | 2019 |
| Tocilizumab (Acetmra®) | 2019 |
| Abatacept (Orencia®) | 2019 |
| Ranibizumab (Lucentis®) | 2020 |
| Panitumumab (Vectibix®) | 2020 |
| Eculizumab (Soliris®) | 2021 |
| Aflibercept (Eylea®) | 2023 |
| Denosumab (Prolia® and Xgeva®) | 2023 |
| Ustekinumab (Stelara®) | 2023 |
| Certolizumab pegol (Cimzia®) | 2024 |
| Golimumab (Simponi®) | 2024 |
| Darbepoetin alfa (Aranesp®) | 2024 |
| Pertuzumab (Perjeta®) | 2024 |
| Ipilimumab (Yervoy®) | 2025 |
| Natalizumab (Tysabri®) | 2027 |
| Etanercept (Enbrel®) | 2028 |
Footnote
1. Based on sales reported by respective manufacturers (1. Humira—Abbvie ($20.39B), 2. Keytruda—Merck ($14.38B), 3. Eylea—Aflibercept ($8.36B), 4. Stelara—Johnson & Johnson ($7.94B), 5. Opdivo—Bristol-Myers-Squibb ($7.92B), 6. Enbrel—Pfizer/Amgen ($6.37B), 7. Avastin—Roche ($5.32B), 8. Trulicity—Eli Lilly ($5.07B), 9. Ocrevus—Roche ($4.61B), 10. Rituxan—Roche ($4.52B).
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.

