With the recent awarding of the 2023 Nobel Prize in Physiology or Medicine to Dr Katalin Karikó & Dr Drew Weissman, for their discoveries that enabled the development of effective messenger RNA (mRNA) vaccines against COVID-19, vaccines are clearly at the forefront of biotechnology innovation1.
This article explores recent developments in the field of vaccines, and in particular, considers important patent filing trends in this dynamic and fast-moving field. As the article explains, patenting vaccine technologies has shown significant growth in recent years, particularly in the field of RNA- and DNA-based vaccines.
Vaccine modalities
Since the first vaccine consisting of cowpox pus was invented by Edward Jenner in 1796 to treat smallpox, various different vaccine technologies have been developed. One established type of vaccine technology, known as "live-attenuated vaccine" (e.g. used in the combined measles, mumps and rubella (MMR) vaccine), introduces a weakened (i.e. "attenuated") version of a live virus to the body, so that a defensive immune response is stimulated against the virus, but without causing disease. Another highly effective vaccine modality, called "inactivated vaccine", one example of which is the influenza vaccine, consists of an inactivated (i.e. killed) virus, which is strong enough to create a defensive immune response, but incapable of causing disease. There are also so-called "subunit vaccines", which present a small part of the virus, such as a viral outer coat protein, to the body, and virus-like particles (VLPs), which are molecules closely resembling viruses, but which do not contain the genetic material of the virus, and so cannot cause infection.
Other vaccine modalities involve delivering genetic material, such as DNA or messenger RNA (mRNA), and so the cells of the person receiving the vaccine can make the viral proteins that ultimately stimulate the immune response and produce protective antibodies. DNA vaccines (e.g. the Oxford University-AstraZeneca COVD-19 vaccine, "ChAdOx1 nCoV-2019") deliver the coding sequence, often via a viral vector, which the host cells transcribe into the encoded protein, whereas for mRNA vaccines (e.g. the Pfizer-BioNTech COVID-19 vaccine), the transcription process has already been performed in the laboratory.
mRNA vaccines and Kariko & Weissman's work
Prior to Karikó & Weissman's research, mRNA vaccines were not seen as a practical approach, because the artificially synthesised mRNA would cause the host's own immune system to launch a self-defensive inflammatory attack before the encoded viral protein could be produced.
Karikó & Weissman overcame this problem, however, by inventing an elegant way of modifying the mRNA to make it less inflammatory, so that it could produce its encoded protein, and thereby induce a protective immune response. They found that replacing the base, uridine, one of RNA's building blocks, with a modified version, pseudouridine, could dampen the unwanted inflammatory response, and so stimulate an immune response by producing protective antibodies to the protein encoded by the RNA. This work that led to Karikó & Weissman's 2023 Nobel Prize has been protected through a series of patents in multiple jurisdictions, based on the PCT application (WO 2007/024708), entitled "RNA Containing Modified Nucleosides and Methods of Use Thereof", filed in 2006.
Patent landscape of vaccines since Covid
With COVID-19 highlighting the importance of vaccine technologies, it begs the question as to whether there has been an increasing upward trend in vaccine innovations and their corresponding protection, since 2020.
A patent search conducted on Espacenet (the European Patent Office's online patent search tool) revealed that there have been 17,418 published patent applications between October 2020 and October 2023 in the field of vaccines (IPC Classification A61K 39/00, "Medicinal preparations containing antigens or antibodies"). This is an increase on the previous three-year period (October 2017 to October 2020), in which 14,328 patent applications in this classification were published, and a significant increase on the three-year period prior to that (October 2014 to October 2017), in which just 9356 applications were published.
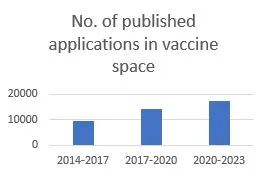
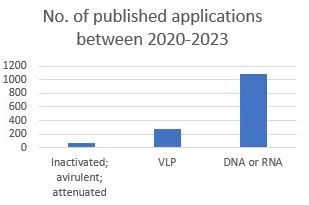
Given the success of the COVID-19 mRNA vaccine, it is also interesting to consider the number of publications for different types of vaccine technologies. An Espacenet search revealed that between October 2020 and October 2023, there have been 62 published patent applications relating to inactivated, avirulent and attenuated vaccines (IPC A61K 2039/5252 & A61K 2039/5254), 270 publications relating to VLPs (IPC A61K 2039/5258), and 1081 publications covering DNA or RNA vaccines (A61K 2039/53).
Additionally, a recent report published by the EPO on mRNA technologies2, highlights the significant growth in the dynamic field of mRNA vaccines, compared to all fields of technology combined over the past decade. As shown in the graph below, the increase in growth in the field of mRNA-based vaccines (the red line) is far above average when compared to the growth for all fields of technology (the bars).
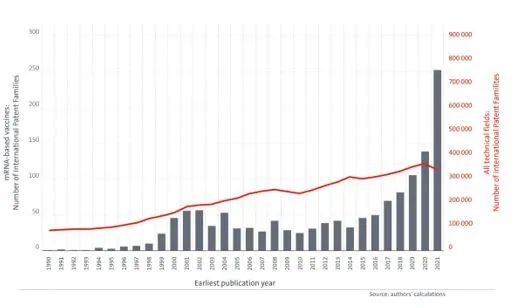
These findings illustrate the breakthrough the mRNA COVID-19 vaccine represents for further nucleic acid vaccine technologies, and there are no signs that developments in mRNA vaccines will lose momentum. As we enter this new era of mRNA vaccines, we are likely to see their application extend beyond just the prevention of viral infections, but also to the treatment of other diseases, including parasitic or bacterial infections, and cancer.
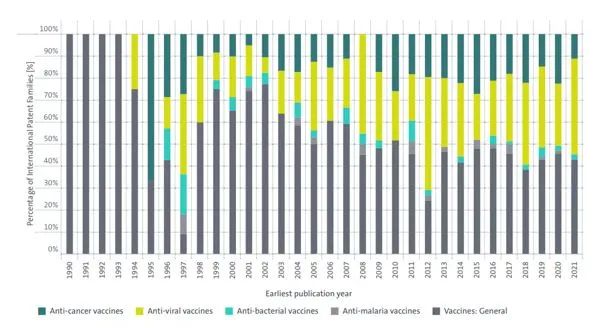
This trend is already emerging, as illustrated in the graph below (taken from the EPO report on mRNA technologies2), which looks at the percentage of International Patent Families since 1990, for important groups of mRNA vaccines: anti-cancer vaccines, anti-viral vaccines, anti-bacterial vaccines and anti-malaria vaccines. As illustrated below, the percentage of inventions with a focus on these specific types of vaccines has grown significantly, with a high percentage addressing anti-viral and anti-cancer vaccines in recent years.
With the significant growth of vaccine innovations in recent years, it will be exciting to see how these trends develop over the next few years.
Venner Shipley has attorneys who specialise in drafting and prosecuting patent applications in the vaccine space, and we have handled a number of vaccine patent cases before the EPO Opposition Division and Boards of Appeal. Our experience covers antibacterial, antiviral and antifungal vaccines, harnessing a wide range of different modalities, such as live-attenuated or inactivated virus/bacterium, the use of virus-like particles (VLPs), glycoconjugates, DNA, RNA (including the highly innovative self-amplifying RNA platform), and vaccines comprising immune cells, such as dendritic or NK cells. We also have experience in applications relating to novel RNA modifications for improved vaccine stability, and carriers, such as lipid nanoparticles and liposomes, for improved vaccine delivery.
Footnotes
1. Press release: The Nobel Prize in Physiology or Medicine 2023
2. Patent insight report – mRNA technologies – October 2023
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.


