- within Food, Drugs, Healthcare and Life Sciences topic(s)
- with Senior Company Executives, HR and Inhouse Counsel
- in United Kingdom
- with readers working within the Pharmaceuticals & BioTech industries
We first reported on the new EU regulatory framework governing medicinal products in April 2023. Since then, following industry feedback and careful scrutiny of the draft package, a lot has changed. This upcoming 3-part blog series will cover the expected timeline to adoption and the current status of the reforms to the EU regulatory framework, including reforms to RDP and orphan market exclusivity; the process for marketing authorisations and supply and shortages of medicinal products in the EU; and bolar exemptions.
Background
The Commission's proposal, first published on 26 April 2023, includes a new Regulation 2023/0131 and Directive 2023/0132 to replace the current EU regulatory framework for medicinal products, with the proposed aim of making medicines more available, accessible and affordable while simplifying the legislation and promoting innovation. This is the first major review of the pharmaceutical legislation since 2004.
On 10 April 2024, the European Parliament adopted its initial position on the European Commission's proposed amendments to the EU regulatory framework governing medicinal products. Following the June elections, the newly elected Parliament published its progress report for presentation to the Council of the EU on 18 November 2024.
Timeline to adoption
The proposed regulatory framework is now awaiting the Council of the EU's first reading. Once the Council has adopted its position, trilogue negotiations will then take place between the Commission, the Parliament and the Council to decide on the proposal's adoption.
It is not expected that the proposed regulatory framework will make it through to adoption before 2026. Following adoption of the final text, there will be an 18-month transition period after which the new Regulation will apply. Member States will then have 18 months to transpose the new Directive into the Member State's national law.
Current status of reforms to the EU regulatory framework
The Parliament's proposed amendments to the Commission's proposal – recently published in the Official Journal of the European Union (OJEU) on 13 March 2025 (C/2025/1328 and C/2025/1329), introduce several key changes. We focus in this first blog in the series on the reforms proposed by Parliament to regulatory data protection, market protection and the orphan medicine regime.
- Regulatory data protection
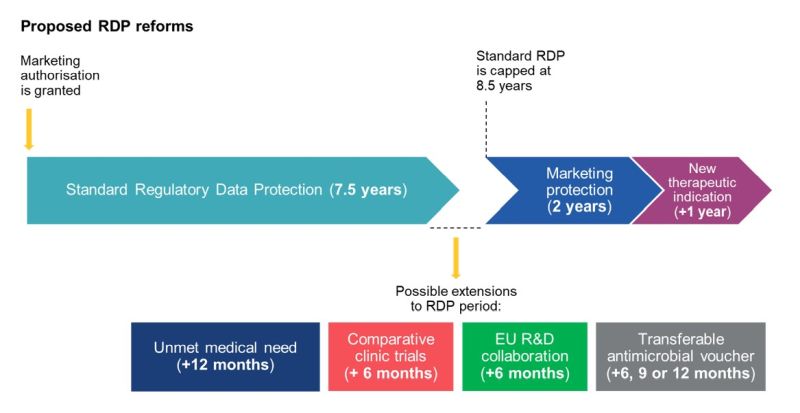
The period of regulatory data protection for new medicinal products is currently a one-size-fits-all regime lasting 8 years, followed by 2(+1) years of marketing protection.
The Commission's proposal to reduce the standard period of regulatory data protection to 6 years has been revisited by Parliament following industry pushback, and the baseline regulatory protection has now been increased to 7.5 years. However, the total duration of regulatory data protection can be extended by a max. of 1 year under certain circumstances, the maximum period being capped at 8.5 years.
As to the circumstances in which the 7.5-year baseline protection can be extended to 8.5 years, Parliament has adopted the Commission's potential additional periods of data protection, with some amendments, including:
- An additional 12 months for products addressing "unmet medical need" (instead of 6 months proposed by the Commission).
- A new additional 6 months if a significant share of the product's research and development takes place in the EU and, at least partly, in collaboration with an EU public entity. A "significant share" has not been defined.
- An additional 6 months if the marketing authorisation (MA)
holders conduct comparative clinic trials for the product (as
proposed by the Commission).
The Commission's proposals for an additional 2 years of data protection for medicinal products launched in all Member States and an additional 1 year of data protection for a new therapeutic indication have been rejected by Parliament.
- Market protection
The standard additional 2-year period of market protection remains unchanged. As under the current system, an additional 1-year extension to market protection can be obtained, if MA is obtained for an additional therapeutic indication during the data protection period which provides significant clinical benefit compared to existing treatment – this applies instead of the Commission's proposal for an additional 1 year of data protection for a new therapeutic indication.
- Transferable exclusivity voucher system
The Commission's proposal for an additional 12 months of transferable data protection for MA holders that develop priority antimicrobials has been amended to 6, 9 and 12 months for holders which develop antimicrobials with "medium", "high", or "critical" priority rankings, respectively.
The transferable data exclusivity vouchers can be transferred once to another MA holder, or retained by the applicant, and can be used only once by the holder in relation to one centrally authorised medicinal product (which can be the priority antimicrobial or another medicinal product). The voucher extension period will not apply to products that have already benefitted from the 8.5 year maximum regulatory data protection period. The voucher will cease to be valid if it is not used within five years of the date it was granted.
.jpg)
- Orphan medicines regime
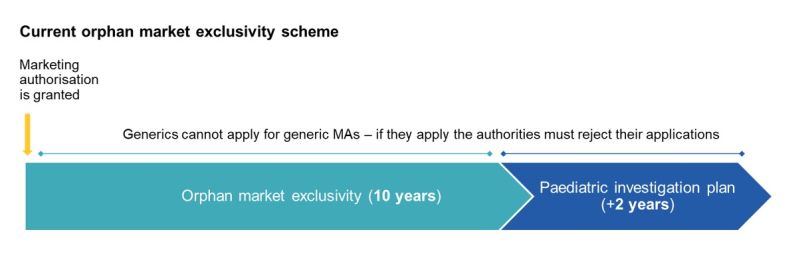
Under the current regulatory framework, orphan medicinal products benefit from 10 years of market exclusivity, with a possible 2-year extension available where a compliant paediatric investigation plan is completed.
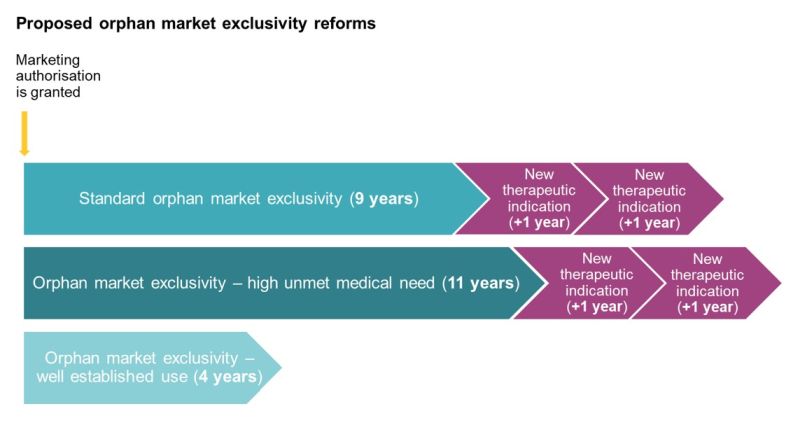
The reforms adopted by Parliament have maintained the Commission's proposal that the standard period of market exclusivity be reduced to 9 years. However, Parliament proposes that orphan medicinal products addressing a 'high unmet medical need' will benefit from an additional 2 years of market exclusivity (replacing the additional 1 year proposed by the Commission) – i.e. a total of 11 years.
The new regime also provides for two possible extensions of 1 year each to the period of market exclusivity. This applies for standard orphan medicinal products and products addressing a high unmet medical need which obtain an MA for new therapeutic indications for a different orphan designation at least 2 years before the end of the market exclusivity period. However, Parliament has maintained the Commission's proposal that separate periods of market exclusivity (i.e. of 9 or 11 years) for different orphan designations will not be available for the same product.
Therefore, standard orphan medicinal products and products addressing a high unmet medical need can benefit from up to a total of 11 years (9+1+1) and 13 years (9+2+1+1) exclusivity respectively.
In comparison, orphan medicinal products based on well-established use will only benefit from market exclusivity of 4 years (replacing the 5 years proposed by the Commission).
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.




