- within Government, Public Sector, Energy and Natural Resources and Criminal Law topic(s)
- with readers working within the Law Firm and Construction & Engineering industries
The Pharmacovigilance Programme of India, (PvPI) was first introduced in the year 2010 to improve safety of Indian population by monitoring drug related Adverse Drug Reactions (ADRs) collected via ADR monitoring centers across the country and the data was further sent to National Coordinating Centre (NCC)-PvPI.
NCC-PvPI receives suspected ADRs from all stakeholders, including Marketing Authorization Holders (MAHs), and reviews the safety information on a regular basis. It supports the Central Drugs Standard Control Organization (CDSCO) for further regulatory actions in the following manner:
- Signal Review Panel (SRP) of NCC-PvPI analyses the suspected ADRs and identifies India specific drug safety signals and recommends its findings to CDSCO for appropriate regulatory action.
- Subject Expert Committee (SEC) of CDSCO reviews the SRP recommendations, and if agreed with SRP, the SEC recommends the same to CDSCO for implementation or for inclusion of the ADRs in prescribing information (package inserts and drug safety labels). In case the SEC is not satisfied with SRP recommendations, it asks the NCC-PvPI to submit more Individual Case Safety Reports/signals to support their recommendation.
- CDSCO after considering recommendation of SEC, requests all State/UT Drugs Controllers to instruct all the manufacturers licensed for the said product/drugs to implement the same in prescribing information.
At present, a total of 47 drug safety signals, as identified and forwarded to CDSCO by SRP1, are categorized on the basis of their current regulatory status:
- Action taken by CDSCO
- Information sent to CDSCO by SRP, and is in process
- SEC recommended to CDSCO
1. Action taken by CDSCO: CDSCO requested all State/UT Drugs Controllers to instruct all manufacture of said product in their jurisdiction to implement the specified recommendations:

2. Information sent to CDSCO by SRP, and is in process:

3. Subject Expert Committee recommended CDSCO to direct the concerned manufacturers to incorporate following changes in package inserts/drug safety labels:
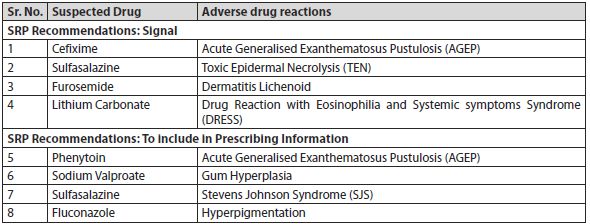

Note - The NCC-PvPI also publishes the preliminary analysis of ADRs at regular intervals in the form of Drug Safety Alert to inform the health care professionals, patients and consumers to closely monitor the possibility of ADR associated with the suspected drug, and if such reaction is encountered then report it to the NCC-PvPI.
Footnote
1. https://ipc.gov.in/images/pdf/PvPI_Recommendations_to_CDSCO.pdf
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.


