- with readers working within the Retail & Leisure industries
- within Intellectual Property, Antitrust/Competition Law and Employment and HR topic(s)
- in Australia
In the field of patent applications, any oversight of details may affect the outcome of the application. Particularly in the biological field, the Sequence Listing is usually a core application document, and its compliance and accuracy directly determine the success or failure of a patent application.
Recently, on April 30, 2025, the China National Intellectual Property Administration (CNIPA) released the Draft Revised Patent Examination Guidelines (for Public Comment), which specifically mentions the calculation rules for the application surcharge related to Sequence Listings. The revisions include two aspects:
In Section 1 of Chapter II of Part V, it is stipulated that "for a Sequence Listing in computer-readable form submitted in compliance with the specified format, the number of pages shall not be counted"; and the provisions on the calculation of fees for Sequence Listings exceeding 400 pages in Section 7.3 of Chapter I of Part III have been deleted.
It should be noted that for regular national applications submitted in paper form, the application surcharge shall still be calculated based on the number of pages of the paper-based Sequence Listing. Additionally, it is important to highlight that the above-mentioned revisions have not yet been implemented and are currently only open for public comments.
Faced with these complex policy changes, are you looking for a professional and efficient solution? We will provide you with a detailed interpretation to help you avoid application risks and make every effort to save costs!
What is the sequence listing in a patent application?
A Sequence Listing is a standardized document that records nucleotide/amino acid sequences. For an invention patent application containing one or more nucleotide or amino acid sequences, the specification shall include a Sequence Listing that complies with the provisions of the patent administrative department of the State Council (Rule 24 of the Implementing Rules for the Patent Law).
According to the provisions of the Guidelines for Patent Examination, when an invention involves a nucleotide sequence consisting of 10 or more nucleotides, or an amino acid sequence of a protein or peptide consisting of 4 or more L-amino acids, an electronic file of the Sequence Listing that meets the requirements of the China National Intellectual Property Administration (CNIPA) shall be submitted. This file contains complete information on biomolecular sequences and relevant annotations, and serves as the core presentation form of the patent technical solution in the biological field. For example, in patent applications for technologies such as gene therapy and protein drug research and development, the key gene or protein sequences recorded in detail in the Sequence Listing are important bases for defining the scope of patent protection. A standardized Sequence Listing not only helps examiners accurately understand the technical solution, but also is a necessary condition for the patent to obtain authorization.
Failure to submit a Sequence Listing or submission of one that does not conform to the required format may result in the rejection of the application on the grounds that the specification does not disclose the invention sufficiently (Section 9.2.3 of Chapter X of Part II of the Guidelines for Patent Examination).
What are the requirements and charging standards for sequence listing files specified by the China National Intellectual Property Administration (CNIPA)?
Starting from July 1, 2022, for patent applications filed with the China National Intellectual Property Administration (CNIPA), the electronic file of the Sequence Listing must strictly comply with the requirements of the WIPO ST.26 standard:
2.1 Mandatory Format Requirements
Sequence Length:
Nucleotide sequences: ≥ 10 unbranched units
Amino acid sequences: ≥ 4 unbranched units
Character Limit: A single sequence must be ≤ 15,000 base pairs (sequences exceeding the limit shall be split with an accompanying explanation).
2.2 Current Fee Standards
When the nucleotide and/or amino acid Sequence Listing is submitted as a separate part of the specification:
If the total number of pages of the Sequence Listing plus the specification exceeds 30 pages, an official fee of RMB 50 (about USD 7) per additional page applies;
If the total number of pages exceeds 300 pages, an official fee of RMB 100 (about USD 14) per additional page applies;
If the Sequence Listing (as a separate part) exceeds 400 pages, the Sequence Listing shall be calculated as 400 pages for fee purposes (Section 7.3 of Chapter I of Part III of the Patent Examination Guidelines).
2.3 Common Reasons for Rejection
Failure to pass XML Schema validation (accounting for 68%);
Inconsistency between sequence identifiers and the specification (accounting for 22%).
What Are the Challenges in Electronic Submission of Sequence Listings?
As mentioned above, Sequence Listings must strictly comply with the WIPO ST.26 standard, and the files must be in XML format. For a single sequence, only unbranched nucleotide sequences with no fewer than 10 nucleotides or unbranched amino acid sequences with no fewer than 4 amino acids meet the requirements.
In addition, during electronic submission, the official system stipulates that the number of characters of a single sequence shall not exceed 15,000. Once the number of characters exceeds the limit, your application will be returned, and you will only be able to resubmit it in paper form. This not only wastes time but may also cause you to miss the optimal application window! If the length of the specification increases significantly due to the Sequence Listing, additional surcharges will be incurred accordingly.
During electronic submission, you also need to pay attention to details such as the labeling specifications for CDs or floppy disks, as well as the simultaneous submission of PDF format files.
Strategy for Sequence Listing Submission: How to Create and Submit a Sequence Listing Efficiently, Accurately, and Cost-Effectively?
4.1 Authoring Tools
It is recommended to use Biopython or PatentIn 3.6 to generate ST.26-compliant files, avoiding tag nesting errors caused by manual editing.
4.2 Cost Control Measures
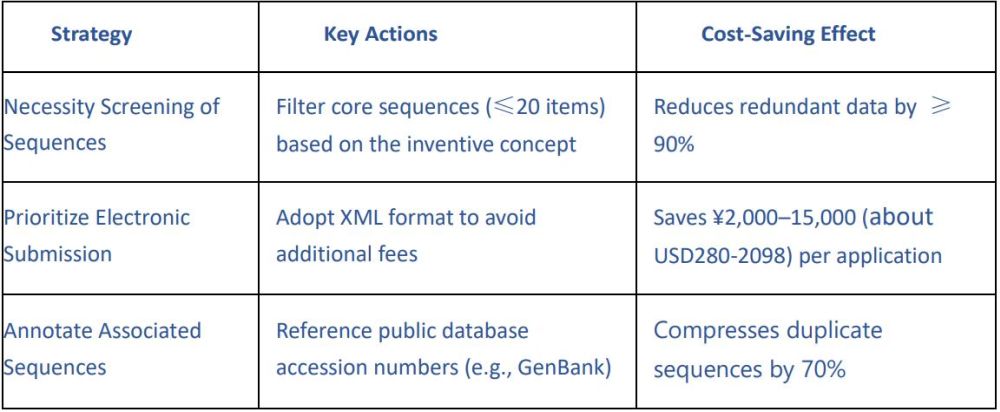
Experienced Team + Extensive Case Portfolio: Let Expertise Secure Your Success
Patent application is a "professional battle." We bring together the industry's top patent agents and seasoned attorneys in the biological field to provide meticulous refinement of sequence listing content and rigorous format checks. From seamless collaboration with patent specifications to professional strategies for responding to patent office examinations, we offer comprehensive, one-stop services. Additionally, our extensive database of successful cases in the same field helps you uncover key techniques, avoid common pitfalls, and safeguard your innovative achievements!
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.


