- with readers working within the Media & Information industries
Appeal of Examiner's Final Rejection of claims 18-20 reversed by PTAB based on calculation of an effective human dosage from a "minimal risk of toxicity" dosage for a Cebus monkey rather than a calculation of "minimum pharmacologic activity in humans."
Ex parte Do Kyu Pyun and Kyoung Jin OO
Application 17/859,603
Independent claim 18 is representative of the claims on appeal and recites:
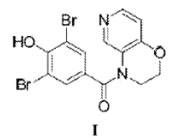
18. A method for treating hyperuricemia, gout disease, nephritis, chronic renal failure, nephrolithiasis, uremia, urolithiasis, or a disease associated with uric acid, comprising: orally administering once daily a pharmaceutical composition which comprises as an active ingredient a compound of the following Formula I, or a pharmaceutically acceptable salt thereof or a hydrate thereof at a human dose of greater than 2 mg to 10 mg or less based on the free base of the compound of Formula I to a human subject in need thereof:
In a Final Action (1), the Examiner rejected claims 1-20 under 35 U.S.C. Section 103 as being unpatentable over Ahn '467, Ahn 2016, Nair, and de Souza.
The Examiner found that Ahn '467 teaches the claimed compound as its Compound 4 and its HCL salts as Compound 33, as well as methods of synthesis, and oral administration of this family of compounds in dosages of 1-200 mg/day. The Examiner pointed to an example in which Cebus monkeys were given a dosage of 3.75 mg/kg to test for uricosuric effectiveness.
The Examiner found that Ahn 2016 teaches the claimed compound, which it refers to as UR-1102 and that UR-1102 administered at 3mg/kg/day demonstrated both better systemic exposure and significantly greater plasma urate-lowering effectiveness than benzbromarone at equivalent or higher doses. Ahn 2016 explained that there are significant differences between species in terms of urate metabolism and that the inhibition of renal urate transporter 1 by UR-1102 would be expected to cause significant urate-lowering in hyperuricemia patients. The Examiner found that Ahn 2016 further teaches that UR-1102 would be expected to show a much more significant effect in human patients than the decreases in plasma urate level achieved in Cebus monkeys.
The Examiner next pointed to Nair, which teaches a method for calculating an initial human equivalent dosage HED based upon the no observed adverse effect level ("NOAEL") of a drug, as derived from preclinical, animal-based, toxicological studies. Using Nair's formula, the Examiner calculated the HED when the average mass of a Cebus monkey is 1.5 kg and the average mass of an adult human is 60 kg based on a dosage in a Cebus monkey of 3.75 mg/kg (from Ahn '467). Using these variables, the Examiner calculated a HED for a human of 1.11 mg/kg or, for a 60 kg human, 66 mg. Dividing by the safety factor of 10, the Examiner calculated a final dosage of 6.6 mg which is within the claimed range of 2 mg to 10 mg and stated that a person of ordinary skill in the art would have been motivated to administer 2 to 10 mg to a human, because one would expect there to be a more significant effect of the drug in human patients, as described by Ahn 2016.
The Appellant argued that: (a) the cited prior art does not disclose all elements of the method of Claim 18; (b) the Examiner has misinterpreted the art; and (c) the cited art would not have guided a skilled artisan to a human dose of greater than 2 mg to 10 mg or less of Formula I, as Nair teaches that the calculated dose where the HED adjusted by the safety factor is a dose with "minimum risk of toxicity," instead of one with "minimum pharmacologic activity in humans." Appellant contends that this initial safe dose would not necessarily have a therapeutic effect. Summarizing, Appellant argues that the Examiner's calculations are either irrelevant to the claimed method or inconsistent with the teachings of Nair.
The PTAB considered claim 18 to be dispositive of the appeal. The PTAB affirmed the Examiner's calculations but stated Nair teaches that the HED is meant to be a dose in humans equivalent to that used in animal studies at which there were no observable adverse effects. With the Examiner's calculated HED of 66 mg, per Nair, the claimed compound would therefore have to be at least six to ten times as effective in humans than in Cebus monkeys to be as efficacious in treating uric-acid related diseases at the claimed doses. The PTAB concluded, based upon the evidence of record, and despite the speculative predictions of Ahn 2016, that a person of ordinary skill in the art could not have reasonably expected a ten-fold increase in the efficacy of the claimed compound in humans compared to Cebus monkeys.
The PTAB reversed the rejection of claims 18–20 as unpatentable under 35 U.S.C. § 103.
A copy of the Decision can be found HERE.
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.


