Holding: In Genentech, Inc. v. Aurobindo Pharma Limited, No. 19-cv-78-RGA, 2020 WL 6144696, (D. Del. Oct. 20, 2020), United States District Court for the District of Delaware (Judge Richard G. Andrews) held that the term "Grade 2 abnormality in one or more biomarkers of liver function," as recited in three asserted patents, U.S. Pat. Nos. 7,566,729; 7,635,707; and 8,592,462, is not indefinite, and adopted Plaintiffs' proposed claim construction.
Background: Plaintiffs Genentech and InterMune brought a Hatch-Waxman patent infringement action against various generic pharmaceutical companies. Id. at *2. The case turned on the construction of the claim term "Grade 2 abnormality in one or more biomarkers of liver function," as recited in various asserted claims of the three asserted patents. Id.
A representative claim is below.
- A method of administering pirfenidone to treat a patient with idiopathic pulmonary fibrosis (IPF), said patient having exhibited a grade 2 abnormality in one or more biomarkers of liver function after pirfenidone administration, comprising
(a) administering to said patient pirfenidone at doses lower than 2400 mg/day for a time period, followed by
(b) administering to said patient pirfenidone at doses of 2400 mg/day or 2403 mg/day.
See claim 1 of the '729 patent (emphasis added).
District Court Discussion:
Plaintiffs argued that "one or more biomarkers of liver function" is defined in the specification as the five biomarkers alanine transaminase (ALT), aspartate transaminase (AST), bilirubin, alkaline phosphatase (ALP), and gamma-glutamyltransferase (GGT). Id. at *3. Plaintiffs contended that the specification consistently identified "Grade 2 abnormalities" with respect to only to these five biomarkers, which are listed in Table 1. Id.
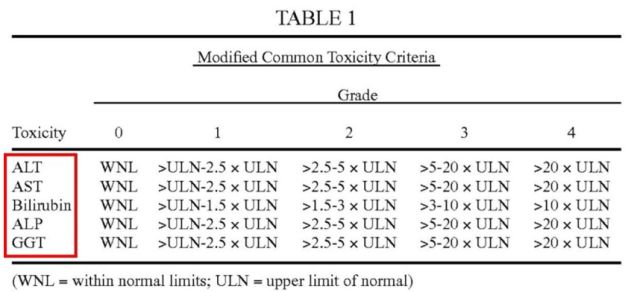
See Table 1 of the '729 patent (emphasis added).
Furthermore, the patent owner wisely identified an a document published by the National Cancer Institute (NCI) for assessing Grade 2 abnormalities. And showing a prescient amount of foresight, the patent owner incorporated by reference the entire NCI document as shown for example in the specification of the '729 patent.

See '729 patent, col. 7, lines 18-23.
Defendants, however, argued that "Grade 2 abnormality in one or more biomarkers of liver function" is indefinite because the phrase has no plain and ordinary meaning and neither the claim language nor the specification indicate the outer boundary of the term. Defendants pointed to several instances in the specification where, "biomarkers of liver function" is allegedly unclear or self-contradictory. Genentech at *4. For example, Defendants cited the following statements recited in the specification:
- "Examples of biomarkers of liver function include, but are not limited to" ALT, AST, bilirubin, ALP, and GGT (see Genentech at *4; col. 6 lines 51-55 of the '729 patent)
- "biomarkers of liver function can exclude gamma-glutamyltransferase" (Genentech at *4; col. 5 lines 12-13 of the '729 patent)
Additionally, Defendants cited other tests that can be used to assess liver function but were not mentioned in the specification. Genentech at *4.
After considering both sides of the arguments, Judge Andrews applied the standard set in Nautilus, Inc. v. Biosig Instruments, Inc., 572 U.S. 898 , 901 (2014) and determined that the term "can be construed with reasonable certainty." Id. The court explained that "claims themselves give important context to the dispute. 'Biomarkers of liver function' does not stand alone. Those words are part of a prepositional phrase that modifies 'Grade 2 abnormality.' That context has to be considered." Id.
The court stated that the patent claims require a "Grade 2 abnormality" and only the five tests—ALT, AST, bilirubin, ALP, and GGT—are defined in the specification as terms of the requisite elevation levels that would constitute such a "Grade 2 abnormality." Id. at *4-*5. Therefore, the court concluded that a person of ordinary skilled in the art would have reasonably understood that only the five tests are defined in terms of a Grade 2 abnormality." "As a result, a POSITA would have been able to determine with 'reasonable certainty' which tests constitute 'biomarkers of liver function.'" Id.
According to the court, the allegedly inconsistent cited statements in the specification "appear[ed] to be nothing more than background" or were inapplicable to the current claim construction because one of the cited statements referred to "liver damage" while the claim to be construed recited "liver function". Id. The court did not consider extrinsic evidence because the relevant "'biomarkers of liver function' [were] apparent from the claims and the specification." Id.
The court therefore determined that the claim term "Grade 2 abnormality in one or more biomarkers of liver function" was not indefinite and adopted Plaintiffs' proposed construction. Id.
Take-Away: This order reminds patent practitioners how application drafting may affect claim construction. In this case, the court found the disputed term definite because five biomarkers were consistently and continuously defined in the specification related to Grade 2 abnormality. And it seemed really helpful that the specification incorporated by reference the test set forth in the NCI document.
Thus, prosecution practitioners should be mindful that statements in the specification can affect claim construction and should strive to use terms consistently in a way that makes the desired meaning clear. And reference to a technical document, including incorporating it by reference, can be so, so helpful.
This order also provides a cautionary tale, which at least before the district court, did not perturb the patent owner's desired claim construction. But we see that use of broadening boilerplate language, such as "Examples of biomarkers of liver function include, but are not limited to ALT, AST, bilirubin, ALP, and GGT", should be carefully considered because, as in this case, it may be used by an alleged infringer to argue a claim is indefinite. And as few of us have the skills of Nostradamus, that argument might be successful for the defense.
It will be interesting to see if this claim construction is appealed. And if so, how will it come out.
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.

