- within Food, Drugs, Healthcare and Life Sciences topic(s)
- with Finance and Tax Executives
The Patent Trial and Appeal Board recently reversed an obviousness finding of (non-signatory) Examiner Audrey Buttice, supported by Supervisory Patent Examienr (SPE) Joanne Hama and Office of Patent Quality Assurance (OPQA) official, Jeffrey Siew, for a method of treating a drug-resistant cancer type with an antibody-drug conjugate (ADC) in Ex part Yonesaka, et al., Appl. No. 16/485,777 (Appeal 2024-003040; Technology Center 1600).
The original claims in the application in question related to a
"therapeutic method for EGFR-TKI-resistant non-small cell
lung cancer, comprising administering an anti-HER3 antibody-drug
conjugate to a subject in need thereof," after
preliminary amendment upon entering the US national stage. These
claims were not amended after an initial and second obviousness
rejection, signed by SPE Joanne Hama, based on WO 2015/155998 A1
(Hettmann), Oncogene 2016, 35,
878-886 (Yonesaka), and optionally OncoTargets Ther.
2016, 9, 6065-6074 (van der Steen), but
after an Advisory Action, the claims were amended with an RCE to
recite "therapeutic method for
osimertinib EGFR-TKI-resistant
non-small cell lung cancer, comprising administering an anti-HER3
antibody-drug conjugate to a human subject
in need thereof."
Osimertinib
The amended claims were rejected over the combination of originally-cited Hettmann, originally-cited Yonesaka, and Oncotarget 2016, 7(49), 81598-81610 (Tang). Similarly to the initial office action, the examiner argued that
[Hettmann] teaches anti-HER3 antibody-drug conjugates (page 228, claim 1, "An anti body-drug conjugate wherein an antitumor compounded .... is conjugated to an anti-HER3 antibody") as a treatment for lung cancer (page 235, claim 38, "the composition ... which is applied to lung cancer ...). The antibody in the antibody drug conjugate taught by [Hettmann] is a human antibody (U1-59; page 19, [0037]) and was tested inhuman lung cancer cell lines (page 20, paragraph 4, Fig.10) which would lead an ordinarily skilled artisan to conclude that the intended subject is a human subject.
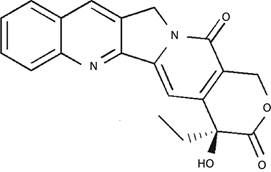
The anti-HER3 antibody-drug conjugate disclosed in [Hettmann] consists of an anti-HER3 antibody (page 4-5, paragraph [0012]) linked to a water-soluble derivative of the drug camptothecin [shown below]
[ ]
]
(page 1, paragraphs [0003] and [0004]). [Hettmann] teaches that the use of this drug is beneficial for cancer treatment as it does not require activation for exerting antitumor effect, has a high inhibitory activity, is active against various cancer cells, and exhibits effect against cells which are resistant to treatment due to expression of a glycoprotein (page 2, paragraph [0005]). [Hettmann] further teaches that the anti-HER3 antibody is an antibody capable of targeting tumor cells and that by using the anti-HER3 antibody in the drug conjugate, the drug is specifically delivered to the tumor cells allowing for reduced dose and less influence on normal cells. [Hettmann] also states that the anti-HER3 antibody is expected to enhance cytocidal effects (pages 4-5, paragraph [0012]).
[Hettmann], however, does not disclose that the anti-HER3 antibody-drug conjugate is used for the treatment of EGFR-TKI [epidermal growth factor receptor (EGFR) tyrosine kinase inhibitor (TKI)]-resistant, particularly osimertinib-resistant, non-small cell lung cancer.
Yoneska teaches a therapeutic method for treating patients with EGFR-TKI-resistant non-small cell lung cancer using patritumab, a monoclonal antibody against HER3 (anti-HER3 antibody) (abstract). Yoneska teaches that the glycoprotein heregulin, the HER3 ligand (page 878, left column, paragraph 1), was upregulated in tissue samples from patients who had TKI-resistant NSCLC (abstract). Yoneska teaches that heregulin is locally secreted and induces HER3 activation through an autocrine mechanism and that HER3 can also be activated by the dysregulation of other tyrosine kinase receptors (page 878, left column, paragraph 1). Yoneska teaches that EGFR-TKIs show robust effects on NSCLC but that cells eventually acquire resistance in approximately half of the cases contributed to both mutations in the EGFR kinase domain as well as HER3. Yoneska teaches that amplification of the MET oncogene can activate HER3, thereby switching on downstream phosphoinositide 3-kinase/AKT survival mechanisms, even in the presence of an EGFR-TKI (page 878, right column, paragraph 2). Yoneska teaches that because of this, targeting HER3 may help prevent the development of cancer cell resistance to EGFR-TKIs (paragraph bridging pages 878 and 879). To test this, Yoneska evaluated the antitumor efficacy of patritumab in NSCLC as a single agent and in combination with established EGFR inhibitors (page 879, left column, paragraph 1). Yoneska teaches that "patritumab can overcome heregulin-dependent EGFR inhibitor resistance in NSCLC in vitro and in vivo and suggest that it can be used in combination with EGFR-TKIs to treat a subset of heregulin-overexpressing NSCLC patients" (abstract).
Thus, the examiner essentially argued that because one antibody-drug conjugate, i.e., anti-HER3 antibody-camptothecin, from the primary reference was indicated to be active against various cancer cells resistant to treatment due to expression of a glycoprotein, such an antibody-drug conjugate would be obvious to use against EGFR-TKI resistance. The examiner acknowledged that the primary and secondary references did not indicate that the EGFR-TKI resistance was osimertinib resistance.
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.


