- within Food, Drugs, Healthcare and Life Sciences topic(s)
- in United Kingdom
- with readers working within the Healthcare and Media & Information industries
- within Food, Drugs, Healthcare, Life Sciences, International Law, Media, Telecoms, IT and Entertainment topic(s)
Summary:
On October 30, 2025 despite HRSA being impacted by the government shutdown, the agency approved 8 drug manufacturers / 9 drugs for participation in HRSA's 340B Rebate Model Pilot Program (340B Rebate Pilot). The 340B Rebate Pilot applies to all Covered Entities (CEs) and it marks a significant shift in how some manufacturers provide 340B pricing to CEs. The Rebate Pilot Program is set to begin on January 1, 2026 even though HRSA missed its October 15, 2025 deadline to approve the initial 340B Rebate Pilot applications. This article contains Polsinelli's key observations and recommendations based on our initial review of the 340B Rebate Pilot.
Program Overview:
HRSA is piloting a back-end rebate model despite CEs and numerous trade associations having raised significant legal, administrative and financial concerns with shifting a discount program to a rebate program. The 340B Rebate Pilot represents the most significant structural test of the 340B Program in decades. Although initially limited in scope, the 340B Rebate Pilot could inform future rulemaking, including broader shifts in the 340B Program. HRSA is shifting 340B resources away from safety-net providers during a time when budgets continue to be tested with other federal program cuts, such as Medicaid reductions. Key elements of the 340B Rebate Pilot include:
- HRSA did not respond to industry stakeholder comments. HRSA has taken the position that it will consider, but has no obligation to respond or act on comments. Given the paradigm shift created by the 340B Rebate Pilot, CEs should continue to provide feedback to HRSA for consideration for the 340B Rebate Pilot.
- HRSA approved eight manufacturer proposals covering 9 drugs contained in CMS's Calendar Year 2026 list of drugs negotiated under the Medicare Drug Price Negotiation Program. Entresto was absent from the 340B Rebate Pilot approvals.
- HRSA did not publish manufacturer 340B Rebate Pilot submissions or HRSA feedback.
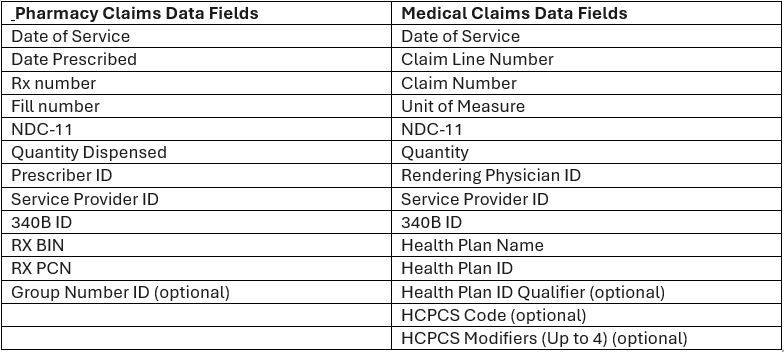
- CEs must continue to purchase potential 340B-eligible drugs from a 340B account but will be charged wholesale acquisition cost (WAC). CEs will submit claims-level data (fields noted below) post-dispense to receive rebates that bring the net price to the 340B ceiling price.
- Manufacturers will be obligated to pay the rebates within 10 days.
- All manufacturers approved opted to use Beacon platform as its software to collect data for manufacturers to approve for rebates. Beacon is managed by Second Sight Solutions who also manages 340B ESP. Manufacturers will also use yet another Beacon tool to implement the IRA. In short, Beacon / Second Sight Solutions will acquire a massive amount of CE data over time.
- Data submissions and rebates will occur at the unit level. Because there are likely thousands of CEs that will submit data, the Beacon platform will have to ingest and accurately process massive amounts of data which puts critical 340B resources at risk for safety-net providers.
- HRSA published Rebate Pilot Program FAQs on its website that fall short on being helpful and understate the massive burden the 340B Rebate Pilot places on CEs. Of note, one FAQ addresses what CEs should do if they do not receive a rebate within 10 days. HRSA suggests that CEs should email HRSA with details, but only after trying to first resolve the issue directly with manufacturers and IT platform vendors, which highlights a lack of understanding of the current 340B environment. We believe it also fails to recognize volume of claims and potential damages at issue if rebates payments are inaccurate, delayed or not paid at all.
Manufacturers and Drugs Approved Effective January 1, 2026

Data Elements Required for Rebate
What CEs Should Do Now?
Advocate for Time, Transparency, and Thoughfulness: It's unclear why HRSA felt the need to create the 340B Rebate Pilot in the first place. Regardless, CEs should continue to support HRSA's oversight of the process and advocate for a more measured approach given the significant shift the 340B Rebate Pilot involves. HRSA should take more time to work with CEs, the intended beneficiaries of the 340B Program, to understand the true implications of a rebate model and implement comments around transparency. It is clear HRSA relied heavily on manufacturer-led discussions regarding data points needed through the 340B Rebate Pilot. CEs must continue to push for a voice on these and other matters.
Assess Inventory Exposure and Model the Financial Impact: Project additional up-front cost of buying the nine products at WAC and assess whether the cost, time, and compliance expenses are worth the 340B benefit for the drugs at issue. Submitting data and pursuing rebates will add incremental administrative, IT/data, accounting and compliance costs. These are very costly products, so we suspect that many will opt to pursue 340B.
Assess Risk Tolerance for Beacon's Terms of Use:Covered Entities need to immediately assess the risks that come with using the Beacon Platform. Beacon requires CEs to accept its terms of use, which favors Beacon and leave CEs with all responsibility and no recourse when something inevitably goes wrong.
Replenish Accumulations in Accumulator Prior to January 1, 2026: Per HRSA's Rebate Pilot Program FAQs, CEs should replenish the amount in their accumulators as soon as possible to avoid a delay in 340B pricing for their replenishments.
Assess Medicaid / Payor Actual Acquisition Cost (AAC) Implications: HRSA has left CEs to fend for themselves in figuring out how to solve the administrative issues the 340B Rebate Pilot will cause with Medicaid agencies that require actual acquisition cost billing. Instead of offering assistance, HRSA has told CEs that they should coordinate with state Medicaid agencies to determine best billing practices, but expect CEs to buy the products at issue from their 340B accounts (at WAC). This will place significant financial risk on CEs if they only receive AAC reimbursement on a drug purchased at WAC. The FAQ suggests CEs will still bill Medicaid at AAC at the point of sale.
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.




