- within Law Department Performance and Real Estate and Construction topic(s)
Below is an excerpt from a recent Manatt Health white paper. Click here to read the full report.
Thanks to breakthroughs in precision medicine, leading scientists have developed a growing number of cell and gene therapies (CGTs) for serious and life-threatening diseases. By addressing the root causes of disease, these transformative therapies can slow, stop, or even reverse disease progression, achieving life-changing results for patients who previously had no hope of effective treatment.
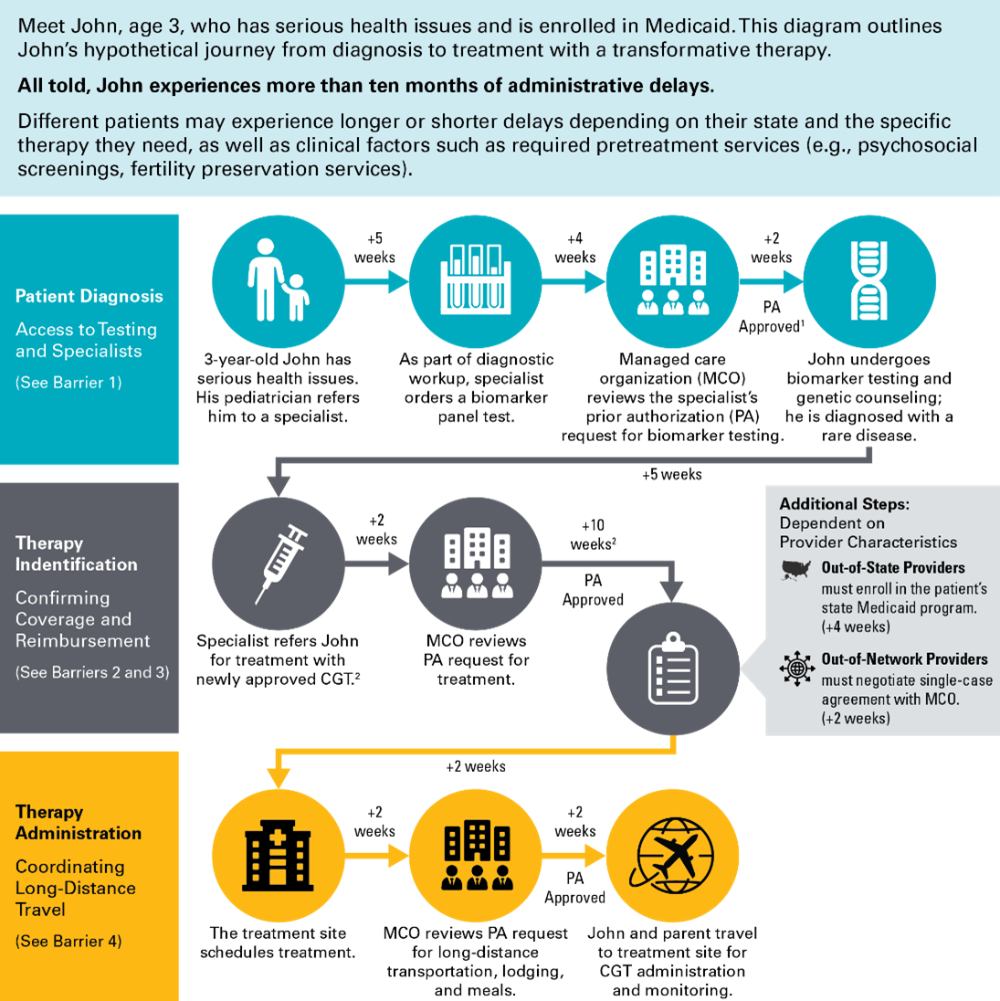
Troublingly, these breakthroughs are not accessible to all. Nationwide, the low-income people covered by the Medicaid program have reduced access to CGTs compared to those covered by Medicare or commercial insurance. On paper, federal law provides that all Medicaid enrollees should have timely access to Food and Drug Administration (FDA)-approved drugs and most specialty care. But in practice, patients and their caregivers can encounter barriers at each step in their journey from diagnosis to treatment.
This paper discusses four key barriers that can impede Medicaid enrollees' access to CGTs, as well as policy solutions to address those barriers at the state and federal level, including:
- Inconsistent access to the testing and specialists needed to confirm the patient's diagnosis and identify the appropriate treatment. Notably, genetic testing is nearly always required to determine whether a patient is eligible for a CGT.
- Unclear, untimely, or unduly restrictive coverage policies for new therapies. Providers are typically unable to take on the significant cost of acquiring, pretreatment processing, and administering a CGT until they receive confirmation that the therapy will be covered for a specific patient.
- Administrative delays between referral and treatment as the provider navigates issues such as prior authorization, reimbursement policies, and cross-state provider enrollment.
- Inadequate support for long-distance travel, without which a low-income patient may be unable to afford the trip to a Center of Excellence for care.
Implementing the solutions identified in the paper will help ensure that these groundbreaking treatments are not just scientific milestones, but accessible lifelines for all who need them.
Findings in the paper were informed by Manatt Cell and Gene Therapy Research Collaborative's survey of relevant Medicaid policies in all 50 states and Washington, D.C., as well as a review of the literature and conversations with diverse stakeholders in the Medicaid ecosystem. Click here to read the full paper and recommendations.
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.





