This is the second part of our three-part article on EPO Enlarged Board of Appeal referral G 2/21. Here, we analyse the technical/legal facts of the appeal behind G 2/21 and provide a digest of post-filed data/plausibility issues in practice. Part I provides a five-minute summary of what you need to know (in a hurry), followed by practical implications for attorneys and applicants to help ensure applications are (plausibly) fit-for-filing and prosecution before the EPO. Part III is a deep-dive into the case law for those looking for the detail, and includes insights that can be gleaned from historical decisions on this area of law.
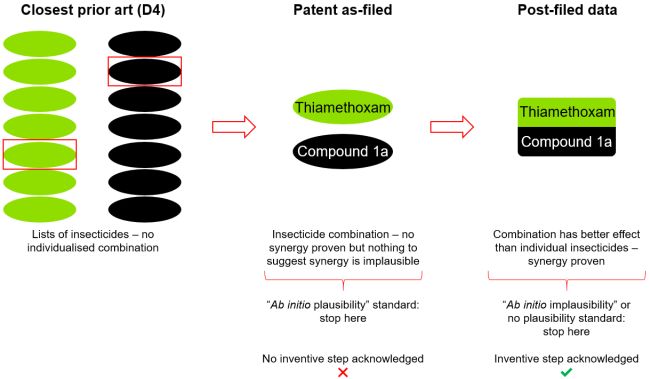
G 2/21 originated in a still-pending EPO Appeal against granted European patent no. EP2484209. This was opposed and a key issue related to whether or not a claimed combination of compounds provided a synergistic technical effect/advantage as compared with the prior art.
The claim in question
Claim 1 of EP2484209 relates to a composition comprising two
insecticidal compounds: thiamethoxam and a "compound of
formula 1a". D4, also relating to insecticides, was identified
as the closest prior art. In D4, thiamethoxam and the compound of
formula 1a were mentioned in two separate lists of insecticides.
However, there was no individualised embodiment/example comprising
both insecticides together, in a single composition. Under these
circumstances, the EPO considers claim 1 of EP2484209 novel (under
the EPO "two-list principle").
Claim 1 therefore is then assessed under EPO inventive step rules
relating to "selection inventions". Here, if the patentee
can prove that there is an unexpected synergy in combining the two
insecticides into a single composition, then inventive step will
generally be acknowledged. In other words, does the composition
provide greater insecticidal activity than the sum of its parts
(the sum of thiamethoxam and compound of formula 1a)?
The opposition
The patent application as filed did contain data allegedly
showing that the claimed insecticides act synergistically against
certain species of moths (Spodoptera litura and
Plutella xylostella). However, an opponent filed their own
data showing that thiamethoxam and chlorantraniliprole (a
representative compound of formula 1a) did not always act
synergistically against these species and that the technical effect
was therefore not achieved across the entire scope of the claims
(i.e. not for all compounds of formula 1a).
The opposition-appeal is being heard by the EPO Technical Board of
Appeal (TBA) in T 116/18. The TBA noted, with respect to the
application as filed, that "arbitrarily combining compounds
known to have insecticidal activity to achieve an alternative
insecticide composition does not require an inventive
step".
However, the patentee filed further data demonstrating synergy for
thiamethoxam and a representative sample of compounds of formula
1a, now against a different species of moth as compared with the
data in the application as originally filed (species Chilo
suppressalis). The TBA acknowledged that "the provision
of an insecticide composition in which the insecticides act
synergistically against Chilo suppressalis" would be
inventive over D4 and acknowledged that the post-filed data (also
known as post-published data) supported this synergy across the
scope of the claims. The only question is whether the post-filed
data can actually be relied upon. These post-filed data were the
only proof of synergy for the insecticidal composition against
Chilo suppressalis.
Hence, the outcome of the EBA referral will dictate whether or not
inventive step can be acknowledged, and therefore whether the
patent can be maintained.
What would each threshold standard being considered in G 2/21 mean for this patent?
The referring TBA in T 116/18 did not comment on how the case in
question would be decided under each of the three standards.
Nonetheless, the fact that the TBA has referred questions to the
EBA implies that the outcome will determine whether they will
accept the post-filed data and therefore whether inventive step
will be acknowledged.
Our predictions, depending on the threshold standard decided in G
2/21, are as follows:
(a) No plausibility standard: low bar
- No consideration of plausibility would be necessary since no data (or other information supporting technical effects/advantages) would be required on filing to support inventive step, so the post-filed data would be admitted.
- Technical problem solved: providing synergistic insecticidal activity.
- Inventive step would be acknowledged.
(b) "Ab initio implausibility" standard (plausibility
is assumed on filing): medium bar
- The referring TBA implies that there were no legitimate reasons to suspect that the claimed composition demonstrated synergistic insecticidal activity against Chilo suppressalis was implausible at the time of filing.
- The post-filed data would therefore likely be admitted.
- Technical problem solved: providing synergistic insecticidal activity.
- Inventive step would likely be acknowledged.
- On 13 October 2022, the EBA issued a preliminary, non-binding, opinion that this was the standard they preferred. However, this position could change during the oral hearing scheduled for 24 November 2022.
(c) "Ab initio plausibility" standard (plausibility
to be proven on filing): high bar
- We suspect the TBA does not consider the application as filed to plausibly show that the claimed composition demonstrated synergistic insecticidal activity against Chilo suppressalis.
- The post-filed data would therefore likely not be admitted.
- Technical problem must be reformulated in a much less ambitious way: providing an alternative insecticide.
- Inventive step would likely not be acknowledged.
Inventive step vs. sufficiency
As a final comment, although G 2/21 and T 116/18 relate to
inventive step, it should be noted that plausibility can also arise
under the umbrella of sufficiency of disclosure.
Here, the facts at issue may not relate to the prior art and
instead arise when the technical effect/advantage is itself
explicitly claimed. This commonly arises where a claim includes a
functional feature or method step (e.g. if a claim is
directed to the treatment of a specific medical condition as a
functional feature). Here, it is vital to ensure that any
explicitly-claimed technical effects or advantages are
well-supported.
As mentioned, a preliminary, non-binding, opinion was issued by the
EBA on 13 October 2022. This indicates that the outcome of G 2/21
may apply only to cases of inventive step
plausibility, and may not extend to sufficiency. This stems from
the fact that the particular issue referred to the EBA relates to
the acknowledgement of inventive step – in the EBA's
view, it may not be appropriate to extend the scope of the referral
to include the context of sufficiency. We therefore expect cases of
sufficiency plausibility to be largely unchanged by G 2/21, and
that EPO Boards of Appeal and Examiners will continue to decide
these on a case-by-case basis.
A notable point raised in some of the G 2/21 amicus briefs
(written opinions by non-parties to the case) is that inventive
step and sufficiency plausibility should be assessed on different
levels. Edgar Wunder's brief gives an interesting legal
justification for this: the wording of Article 56 EPC (inventive
step) refers to an "invention", whereas the wording of
Article 83 EPC (sufficiency) refers to an "application".
Wunder interprets this distinction as meaning that sufficiency of
disclosure should be established at the filing date, while
inventive step can be assessed later in proceedings (and there
should therefore be less of a hurdle for inventive step
plausibility). Perhaps, following G 2/21, this interpretation will
move closer to reality.
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.



