To waive, or not to waive, that is the question.
The earliest identified date of a patient presenting with symptoms of a "novel coronavirus" in Wuhan, China is reported to be 1 December 2019 1. On 11 March 2020 the World Health Organisation (WHO) declared the disease we now know as Covid-19 to be a pandemic 2. Since then, the virus has spread around the world with alarming speed: at the time of writing there have been more than 173 million cases of Covid-19 and sadly more than 3.7 million deaths globally 3. Yet there is cause for hope: on 8 December 2020 Margaret Keenan in Coventry, UK became the first person in Europe to receive a Covid-19 vaccine as part of a mass vaccination program 4. But with a pandemic no one is safe until everyone is safe 5. How can we ensure equitable access to Covid-19 vaccines for everyone; and do patents really stand in the way?
Vaccine development – a global success story
Development of a vaccine to treat a new disease has previously taken upwards of 10 years 6. Yet on 2 December 2020 the UK Medicines and Healthcare products Regulatory Agency (MHRA) gave temporary regulatory approval to a Covid-19 vaccine developed in collaboration by Pfizer & BioNtech, becoming the first country in the Western world to do so 7, less than 11 months after the genetic sequence for SARS-CoV-2 (the virus causing Covid-19) was first published 8. There are currently four vaccines approved for use by both the MHRA and the European Medicines Agency (EMA), developed respectively by Pfizer & BioNtech, Oxford University & AstraZeneca, Moderna and Janssen 9 and at least a further four are under review. This unprecedented achievement has only been possible due to collaboration between the pharmaceutical industry and governments.
All this has come at an eye-watering cost: while much of the cost of development has come from the pharmaceutical industry itself, governments are understood to have provided at least £6.5 billion and not for profit organisations a further £1.5 billion 11. While some companies are predicted to make billions of pounds in profit, others including AstraZeneca have pledged to sell Covid-19 vaccine doses at a price that only covers their overall costs, at least for the duration of the pandemic 12.
Additionally, vaccine development has been undertaken at considerable risk, with no certainty of success. For every vaccine which has received regulatory approval, there are others where the clinical trials have not been promising. A failed clinical trial can prove very costly, not just financially, but also in terms of resources, time and reputation. Even for companies whose vaccines are approved for use, they have been subjected to intense political and media scrutiny, particularly where they have struggled to keep up with promised production levels and where adverse side effects have been reported.
Unequal access to vaccination
Deployment of vaccines has not been evenly spread. Those countries with the highest vaccination rates are predominantly higher-income countries (principally the US and UK with over 51% and 60% respectively of their population having received at least one vaccine dose, with the European Union at 41% and North America as a whole at 39%)13. Worldwide, the same figure is 12%, with extremely low levels of vaccination in parts of Africa, Asia and South America. In many cases those countries home to pharmaceutical companies that have developed vaccines have been ahead of the curve in deploying them, in part due to being first in the queue to place orders 14. The US government has invoked wartime powers through the Defense Production Act to compel private companies to fulfil its contracts ahead of other orders. While stopping short of being an export ban, it has been viewed by some as a ban in all but name. The UK government has been accused of the same, though insists that limited vaccine exports results purely from contractual obligations on AstraZeneca in particular to fulfil its supply agreement with the UK first 15.
Covax
The Covid-19 Vaccines Global Access (Covax) program is a worldwide initiative aimed at ensuring equitable access to Covid-19 vaccines, spearheaded by the WHO 16. Higher-income countries pay for vaccine doses through Covax, while lower-income countries receive vaccines paid for by donations 17. Covax aims to provide two billion doses to people in 190 countries in less than one year, though it is currently falling well short of that ambition 18. WHO Director General, Dr Tedros Adhanom Ghebreyesus has blamed lack of supply under the Covax program on hoarding of vaccine doses by countries with sufficient supplies to cover their entire populations several times over, and bilateral vaccine donations outside of the Covax scheme which risk increasing not reducing vaccine inequity 19.
Vaccine patents
Given the enormous cost of developing vaccines for Covid-19, it is to be expected that companies will seek to protect that investment through the patent system. A search of the Patbase database for the term "Covid-19" reveals over 1342 patent applications filed in 2020 (the picture for 2021 is still emerging), though many of these will cover other inventions relating to Covid-19, for instance test equipment. The leading jurisdictions for patent filings are China, India, US and UK, and the leading patent classifications are shown in the graph below (some applications are classified in multiple fields).
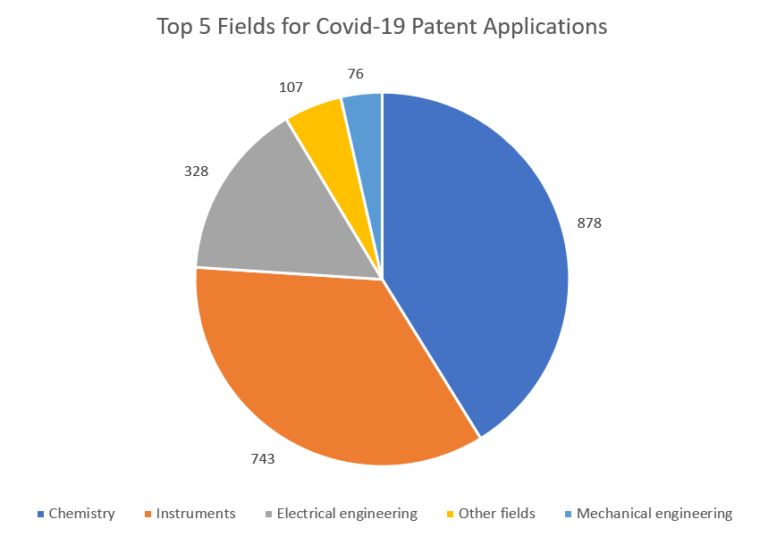
As much of the work to develop Covid-19 vaccines builds upon the experience of developing vaccines for other coronaviruses, it is likely that certain aspects of the manufacturing process will already be protected. In particular, those vaccines based on the use of mRNA (including Pfizer-BioNtech and Moderna) are manufactured using techniques that are known to be patented. Furthermore, patent applications are not normally published for 18 months, meaning the full picture of what aspects of Covid-19 vaccines are protected through the patent system may not be clear for some time.
In the UK, in common with most countries, a granted patent gives the patentee the right to stop others from making, disposing of, using, importing or keeping a product covered by the patent, without permission. Where a patent covers a process such as a manufacturing method, the patentee has the right to stop others from using the process or offering the process for use, or performing the previous acts for a product resulting directly from that process 20.
A patent does not give the patentee the right to make or sell something covered by the patent: of course, for a vaccine marketing approval from a regulatory authority, such as the MHRA or EMA is required. Also, a patented vaccine may still infringe a patent in some underpinning technology, for instance in the manufacturing method. Rather, a patent is a negative right to stop someone else using that invention without permission, for instance via a voluntary licence. This market exclusivity is given for a limited period of time in reward for the investment made in developing a new invention, and in return for disclosing the invention within the patent application in sufficient detail that other people can make and use the invention once the patent has expired.
Are patents to blame for unequal vaccination rates?
If a patent can be used to stop someone else from working the invention, then are patents covering Covid-19 vaccines stifling manufacturing and thus depressing vaccination rates in lower-income countries? This is certainly a point of view which is gaining traction. India and South Africa have led calls for patents covering Covid-19 vaccines to be waived since October 2020 21. They are supported by over a hundred lower-income countries. The argument is essentially that a temporary patent waiver would allow multiple parties to start production sooner, rather than manufacturing being concentrated in a small number of companies holding patents that serve as a block to others.
After initially being opposed to the idea, the US has now joined calls for a patent waiver. US trade representative Katherine Tai said on 5 May 2021 that "The Biden-Harris Administration believes strongly in intellectual property protections, but in service of ending this pandemic, supports the waiver of those protections for COVID-19 vaccines. We will actively participate in text-based negotiations at the World Trade Organization (WTO) needed to make that happen."22 The support of the US was welcomed by Dr Tedros 23, who has long called for a patent waiver that would allow countries to make and sell cheap copies of vaccines that were invented elsewhere 24. Support by the US for a waiver has been followed by Russia and China, while the UK, the EU, Japan and South Korea remain opposed, though seemingly prepared to debate the possibility through the WTO 25.
What would a patent waiver actually mean? Dr Tedros has made clear that in the event of a patent waiver, companies would still receive royalties for the products they manufacture 26, and has called for lower-income countries to be supported to build their domestic manufacturing base. However, there is little or no mention of compensating owners of patents covering Covid-19 vaccines. In this respect, any patent waiver that is negotiated through the WTO may go far further than existing mechanisms to prevent patents from blocking access to essential products. In the UK, during a national emergency (which intuitively would seem to include a pandemic) UK patent law already allows the government to make "use of patented inventions for services of the Crown 27". This permits any government department to do certain acts (including the production or supply of specified drugs and medicines 28) which otherwise would amount to patent infringement, though again the patentee is to be compensated. In essence, this gives the state the power to compel a patentee to licence their invention.
The UK "Crown use" provision is in line with Articles 30 and 31 of the GATT Agreement on Trade-Related Aspects of Intellectual Property Rights (TRIPS Agreement) (1994)29. Any country that is a WTO member is already able to enact legislation allowing for use of a patented invention without having first sought authorisation from the patentee "in the case of a national emergency or other circumstances of extreme urgency". Article 31 still requires that the patentee "shall be paid adequate remuneration in the circumstances of each case, taking into account the economic value of the authorization". It would seem that any WTO member is already in a position to take action if they feel that a patent covering a Covid-19 vaccine was being used to limit the supply of vaccine doses (and implicitly there was untapped domestic manufacturing). However, the patentee(s) would have to be compensated.
It is likely that WTO negotiations to build consensus around the idea of a waiver will be fraught. The EU and Switzerland are opposed, and the G7 have called instead for greater funding of Covax, voluntary licencing and technology transfer to increase manufacturing capacity 30. Western pharmaceutical companies are also strongly opposed. Pfizer argue that they have already taken concerted steps to ensure equitable vaccine distribution, including through tiered pricing, such that lower-income countries are offered doses at cost, and through multi-billion dollar investment in manufacturing to produce four billion doses a year by 2022 31. Further, Pfizer argue that the bottleneck is not manufacturing infrastructure: it is the supply of raw materials, and so a patent waiver could simply divert limited raw materials from one manufacturer to another. Novovax also points to the limited supply of raw materials, and their own efforts to forge technology transfer partnerships to grow manufacturing capacity 32.
A key argument against a patent waiver is that patents incentivise the creation of new inventions, including vaccines, and without the protection a patent affords private industry simply would not make the large investment necessary in the event of a future pandemic. In particular, the mRNA technology underpinning certain vaccines has been expensive and time consuming to develop, and offers hope for vaccines for other diseases. If patents covering those technologies are waived then there is a clear disincentive to undertake similar R&D in the future.
Further, the charge that patents concentrate manufacturing in a small number of companies is undermined by high profile licencing arrangements that have taken place voluntarily, notably the agreement between AstraZeneca and the Serum Institute of India to supply one billion doses for low and middle income countries 33, 34. It is not clear that there are a significant number of pharmaceutical companies with the capability to manufacture Covid-19 vaccines, who are not already doing so, particularly for those companies that have adopted an mRNA vaccine, such as Pfizer-BioNtech and Moderna 35.
The US government has also expressed concern about the risk that a patent waiver might hand sensitive technology to other countries 36. It is hard to see how another company might be in a position to manufacture a vaccine for which any patents have been waived, without being provided with proprietary know-how going beyond what is disclosed by the patents.
Ultimately though, a patent waiver may make no difference 37. Following the support of the US government for a waiver, Moderna have stated that they would still expect countries around the world to continue to buy its vaccine for years to come 38, in view of the significant technological challenge of setting up manufacturing capability, and the need for any new party to conduct their own clinical trials. In short, a patent waiver, or use of existing compulsory licencing powers, is unlikely in the short term to help any country which lacks domestic vaccine manufacturing capability 39.
Footnotes
1. https://www.sciencedirect.com/science/article/pii/B9780128243138000012
3. https://www.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6
4. https://www.bbc.co.uk/news/uk-55227325
6. https://wellcome.org/news/quick-safe-covid-vaccine-development
8. https://twitter.com/WHO/status/1216108498188230657
9. https://www.nhs.uk/conditions/coronavirus-covid-19/coronavirus-vaccination/coronavirus-vaccine/
11. https://www.bbc.co.uk/news/business-55170756
12. https://www.ft.com/content/e359159b-105c-407e-b1be-0c7a1ddb654b
13. https://ourworldindata.org/covid-vaccinations?country=OWID_WRL
14. https://www.ft.com/content/82fa8fb4-a867-4005-b6c2-a79969139119
15. https://www.bbc.co.uk/news/45877605
18. https://www.bbc.co.uk/news/world-56698854
20. https://www.legislation.gov.uk/ukpga/1977/37/section/60
21. https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)32581-2/fulltext
23. https://twitter.com/DrTedros/status/1390037666213900290
25. https://www.nature.com/articles/d41586-021-01242-1
27. https://www.legislation.gov.uk/ukpga/1977/37/section/55
28. https://www.legislation.gov.uk/ukpga/1977/37/section/56
29. https://www.wto.org/english/docs_e/legal_e/27-trips_04c_e.htm
35. https://blogs.sciencemag.org/pipeline/archives/2021/02/02/myths-of-vaccine-manufacturing
37. https://healthpolicy-watch.news/unexamined-prejudices-covid-and-patents/
39. https://www.mpg.de/16579491/patent-protection-vaccines-covid-10-reto-hilty
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.


