In view of the fact that metabolism is the major process by which foreign substances, including drugs are eliminated from the body, or really the sum of many ongoing individual processes by which living cells process molecules and maintain a living state, it is not easy to understand how said processes may biotransform a drug substance into pharmacologically active or inactive metabolites. Therefore, when the subject matter of a patent application deals with a prodrug, it should not be a surprise if as a result of the substantive examination, objections to the patentability of claims reciting prodrugs are issued from the Mexican Institute of Industrial Property (MIIP). In fact, it has become a trend from the MIIP to object the language "prodrug" in the claims of a Mexican application arguing that said language intends to unduly extend the scope of the invention. Arguments from the MIIP include the objection that examples in the specification do not sufficiently support the general language "prodrug" so that the claims become considered as unclear, ambiguous or even speculative.
The systematic way of objecting the language "prodrug" from the MIIP, might lead to mistakenly think that there are specific legal restrictions prohibiting the patentability of prodrugs in the Mexican legislation, but this is not completely accurate as it can be appreciated from the explanation given below.
Although it is comprehensible that prodrug claims must be certainly subjected to special scrutiny, it is to be stressed that the Mexican Law and its Regulations do not include any particular statement dealing with prodrugs. Thus, at the first glance prodrugs should be prosecuted in strict accordance with the Mexican Law, just as it occurs with any other composition of matter, e.g. active agents, formulations, or the like.
The following figure illustrates a clear example of what is considered a prodrug
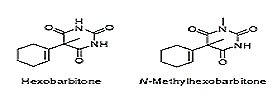
The N-methylhexobarbitone is a prodrug of hexobarbitone which is used as a sedative agent. This prodrug was found to have better permeability characteristics. Prakash Rao Surya A. "Capping drugs: Development of prodrugs". Resonance, February 2003. Page 21.
The term "prodrug" is used in the pharmaceutical field to characterize reversible derivatives of drugs. Originally, these derivatives were designed to eliminate undesirable physical or biological properties of a drug. The most common physical properties are solubility and instability, while undesirable biological properties refer to a very rapid metabolism or a poor bioavailability, which may be related to a physicochemical property of the drug. Generally, when undesirable properties are found in a drug which cannot be eliminated by conventional changes in the formulation or in the route of administration, a reversible derivative (prodrug) can be a solution to solve the problem.
Prodrugs were initially known as "latentiated drugs", "bioreversible derivatives" and "congeners", but now the most commonly accepted term is "prodrugs".
Nowadays, the term "prodrug" is used in a more general sense. One functional definition of prodrug is the following:
"A prodrug is a compound formed by chemical modification of a biologically active compound that will liberate the active compound in vivo by enzymatic or hydrolytic cleavage". A prodrug is not generally classified as a controlled-release dosage form. However, the ability to bioreversibly modify the physicochemical properties of a drug allows better intestinal transport properties and hence influences the drug blood level versus time profile. Thus, prodrugs can be used to increase the strategies for controlled release of a drug.
The following figure illustrates the L-3,4-dihydroxyphenylalanine (Levo-DOPA), which is a prodrug of dopamine. This prodrug improves the membrane transportation (better blood-brain permeation) using amino acid channels for transportation. It is used in the treatment of Parkinson’s disease.
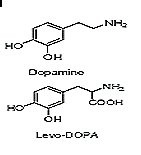
Prakash Rao Surya A. "Capping drugs: Development of prodrugs". Resonance, February 2003. Page 22.
Due to the special property of delivering the active compound in vivo, the prosecution of prodrug claims in a patent application should also be considered as special in the following cases:
1) When a prodrug of a new and inventive active is to be claimed.
It is very common that a patent application, further to claim a new active, claims its pharmaceutically acceptable derivatives such as solvates and salts, as well as its prodrugs.
Recitations of claims comprising the general language "pharmaceutical acceptable salts and solvates" are usually accepted by the MIIP, but it usually tends to reject the term prodrug. The objections usually state that the term "prodrug" is unclear and ambiguous because the specification does not provide enough support for claiming such subject matter, hence the claim is considered as speculative.
Taking into consideration that the specification of a patent application is generally limited to disclose that the invention covers prodrugs of a new active without exemplifying the prodrug production from said new active, the objections to the patentability of the prodrug would seem to be completely justifiable. It is very common to find specifications reciting the following:
"The term "prodrug" as used herein is referred to a compound that is transformed in vivo to yield the new active compound of this invention, for example, by hydrolysis in blood. A deep discussion of such compounds is provided in the following documents……….… which are hereby incorporated by reference".
Moreover, if some possible prodrugs were identified before filing the application, the specification continues reciting:
"Some illustrative and not limitative examples of prodrugs in the context of this invention are derivatives of the new compound such as ester derivatives, N-methyl derivatives………".
Because of the poor information contained in the above lines, in each case the MIIP has determined that said information cannot be considered as sufficient to support the claims directed to any prodrug of the new active, particularly if no examples are disclosed in the specification.
Nevertheless, based simply on the disclosure of said lines and without providing additional information within the specification about the efforts to reach the desired goal, applicants try to protect any prodrug that may be susceptible of being produced from the reading of the invention, but the MIIP considers that this is an unduly way to try to enlarge the protection of the invention to some prodrugs which might require inventive efforts over the disclosure of the specification. This criterion is completely coincident with the current practice in Europe.
Now then, with respect to the MIIP rejections regarding ambiguity, unclearness, lack of support and lack of exemplification, it can be commented the following:
Clarity is a requirement from Article 47 Section III of the Mexican Law which demands that the claims must be clear and concise and must not exceed the contents of the specification. As there is no any legal provision in the Mexican Law requiring examples for all the matter being claimed, when the specification mentions the term "prodrug", it should be assumed that said term is completely supported. Section I of said Article 47 demands that the specification must include the applicant’s best mode known to carry out the invention, when such mode is not clear from the description of the invention.
The clarity rejection of the term "prodrug" would be certainly more plausible if the term is rejected due to a lack of clarity of the specification. Article 47 Section I also states that the specification must be sufficiently clear and complete in order to permit an accurate understanding of same and, if such be the case, to enable its execution by a person possessing skills in the art. However, the term "prodrug" is used as such in the art, so it is deemed that it should be understandable by a skilled person.
The MIIP does not usually object the descriptions of applications because of lack of clarity of the term "prodrug", which is certainly an inconsistency, because Section I of Article 47 is precisely where more statements regarding clearness of the description are quoted. Section III of the same Article simply refers to the claims. Therefore, to reject the term "prodrug" in the set of claims as being unclear without making reference to the description it is more than incongruent, but this actually happens.
Sometimes, the MIIP argues that the term "prodrug" is very broad and involves a huge number of compounds. Nonetheless, broadness is not related to clearness (understandability by a skilled person). In any case, if broadness was the reason to object the term "prodrug", the MIIP should be forced to indicate in a clear and concise manner the number of elements that can be accepted for each term. This action, however, does not seem to be appropriate because it would be considered as arbitrariness from the authority. Furthermore, the definition of prodrug by itself leads to conclude that it does not comprise an undefined number of compounds and that it is limited to only those compounds that reversibly deliver the new active in vivo.
When the specification discloses, although in very general terms, some prodrugs or a particular type of prodrugs obtainable from the new active, the MIIP tends to accept the inclusion of a claim limited to said particular type of prodrugs, but when only some specific prodrugs are claimed and the specification is able to completely support the general term "prodrug", the protection might be extended to those prodrugs being structurally similar to said specific prodrugs. There is no doubt that this criterion gives sense and relevance to the Mexican legislation in patents matter.
Once the new active is conceived, there might be a number of prodrugs obtainable from such active. In order to be part of the invention, the claimed prodrugs should be those that can be obtained without additional information to that contained in the prior art and the detailed description of the invention. Those prodrugs that do not fall within this provision cannot be claimed by the new active application because it constitutes a further invention which has not been sufficiently described or suggested in the specification.
Consequently, and considering the above point of view, it can be established that if there are possibilities that the recitation of the term prodrug in the context of the invention might comprise inventive compounds over the specification without any further limitations, said recitation would result inappropriate on the grounds of Article 47 Section I of the law.
2) When a prodrug of a known active is to be claimed.
Applications trying to protect a prodrug from known compounds do not generally recite any prodrug obtainable from the known compound. Bearing in mind that the description and properties of a known compound as an active have been previously disclosed, some foreseeable prodrugs would not involve an inventive step and could not be considered as patentable (except when they show an unforeseeable property).
If the known compound has already been patented and the owner of said patent applies for a patent claiming a predictable prodrug, this action may be considered as an unduly intention to extend the monopoly of the patent over a known invention. Likewise, if the application is filed by a new applicant, the application may be considered as an unduly approach for excluding other parties to manufacture, use, or sell a non inventive compound. Anyhow, this kind of applications is supposed to be rejected by the MIIP.
On the other hand, if a prodrug is sufficiently distinct from the parent active and if it exhibits unexpected improved properties over the parent drug and over the foreseeable prodrugs, then, based on both, the specification of the known compound and the prior art available of the new application, the claimed prodrug should be considered as patentable. However, and taking into consideration that the description of the known compound will surely correspond to the closest prior art for the new application, it is highly advisable to provide all the information available about the produg in order to support and demonstrate that it is inventive over the known compound.
Conclusions
Based on the above, it can be said that even if the MIIP tends to reject the term "prodrug" in the claims of a new active compound, protection for some specific prodrugs being sufficiently described by the specification could be obtained. Therefore, it is extremely important to consider, when drafting the description of the invention, that the word "sufficiently" shall not simply mean "recitation" or "listing" when such term prodrug, used as a general language, appears in the specification. Although an appropriate section of examples related to prodrugs in the specification would be highly recommendable, it should not be considered as strictly necessary.
However, an unavoidable suggestion is to describe as exhaustively as possible the identified prodrugs of a new active and include any foreseeable prodrugs. Thus, if the term "prodrug" is objected, there will be at least the option of trying to protect the type of prodrugs described, or in the worst case, those prodrugs which have been exemplified. Exemplification does not necessarily mean "testing". The way of obtaining the prodrug may be enough for these purposes.
A prodrug from a known active is susceptible of protection in Mexico if the prodrug shows novelty and inventive step over the description of the known active and the available prior art in this field, which is not generally the same than the prior art for the known compound. This renders the inventiveness a very important point of analysis during prosecution of a prodrug application.
One interesting point of discussion non-addressed here, is if the owner of a drug patent can prevent the use of a prodrug to third parties, disregarding the fact that the prodrug has been patented. Anyhow, it is generally advisable to try to include a suitable language in the drug application to try to cover at least those foreseeable prodrugs. Only in this way there could be chances to challenge manufacturers trying to manufacture, use or sell compounds falling within the spirit of the invention.
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.


