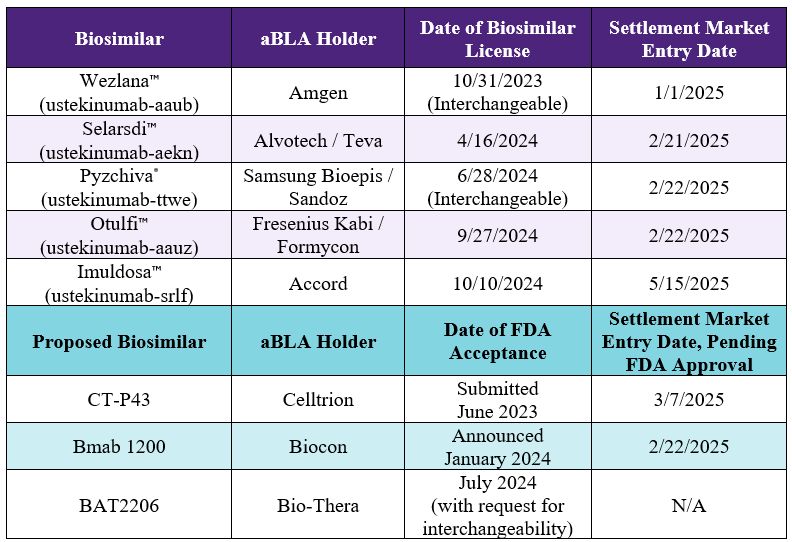
On October 10, 2024, the FDA approved Accord BioPharma's Imuldosa" (ustekinumab-srlf), the fifth biosimilar of Janssen / Johnson & Johnson's Stelara® (ustekinumab). Imuldosa" was developed by Dong-A ST in collaboration with Meij Seika Pharma, and Accord is responsible for commercialization in the U.S. Under an October 2023 settlement agreement, Imuldosa" can launch in the U.S. beginning no later than May 15, 2025.
The table below shows the other approved and pending aBLAs for Stelara® biosimilars as well as their settlement market entry dates. There are no pending patent disputes related to Stelara® biosimilars.
Stelara® was chosen as one of the first 10 drugs for Medicare price negotiations under the Inflation Reduction Act. In August 2024, the Centers for Medicare & Medicaid Services (CMS) announced that as a result of the price negotiations, beginning January 1, 2026, under Medicare Part D, a 30-day supply of Stelara® will cost $4,695, a 66% discount from its 2023 list price of $13,836. However, the anticipated launch of Stelara® biosimilars in early 2025 may affect the status of Stelara® on Medicare's price negotiation list.
Johnson & Johnson reported Stelara® U.S. sales of $6.97B in 2023.
The author would like to thank April Breyer Menon for her contributions to this article.
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.


