- with readers working within the Pharmaceuticals & BioTech industries
- with readers working within the Pharmaceuticals & BioTech industries
- within Immigration, Criminal Law and Cannabis & Hemp topic(s)
A wave of AI investment is helping drive innovation in medtech.
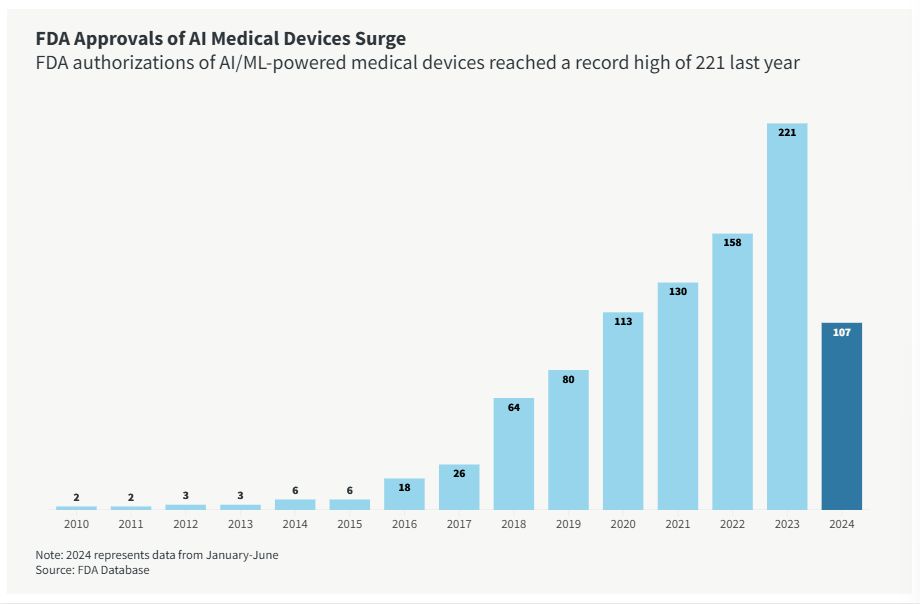
Over the past decade, FDA approvals of artificial intelligence and machine learning (AI/ML)–enabled medical devices have surged. They reached a record high of 221 last year, according to data tracing back to 1995. In the first half of this year, the FDA approved 107 AI-powered medical devices, suggesting they were on pace to nearly reach last year's total.
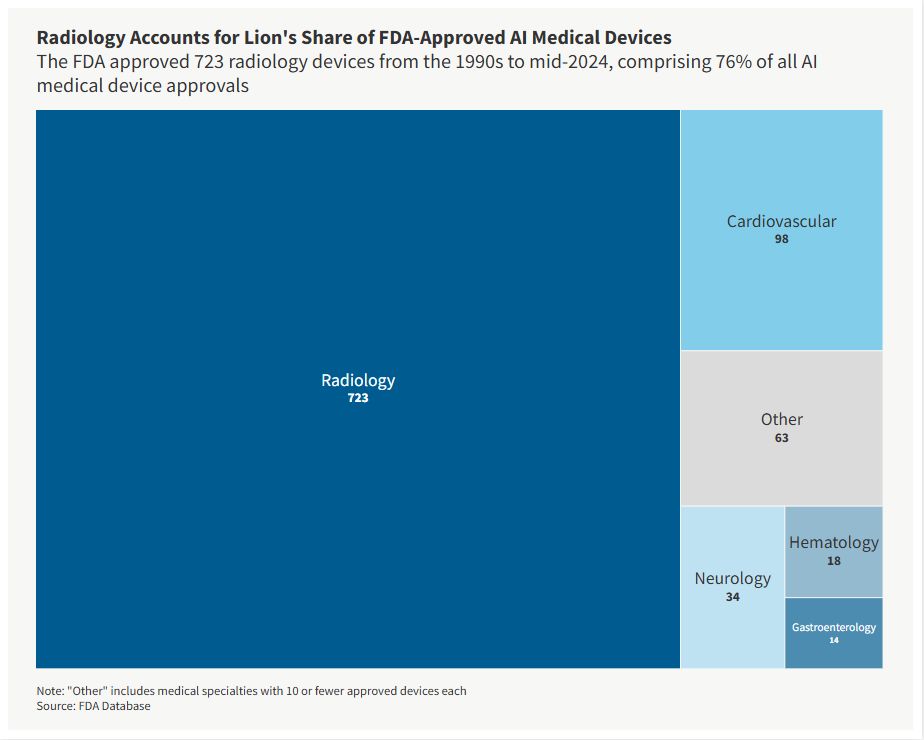
The FDA classifies each medical device under a particular medical specialty. From the 1990s to mid-2024, radiology devices accounted for about 76% of all AI medical device approvals, far more than the runner-up — cardiovascular — which captured 10% of approvals. Immunology, obstetrics and gynecology, and physical medicine logged the fewest approvals.
As AI innovation surges, medtech companies should consider taking steps to protect their privacy while capitalizing on their intellectual property.
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.



