- within Cannabis & Hemp topic(s)
- with readers working within the Retail & Leisure industries
- within Cannabis & Hemp, Antitrust/Competition Law and Employment and HR topic(s)
Introduction
Until June 9, 2022, cannabis and hemp were classified as category 5 narcotics under Thailand's 1979 Narcotics Act and later the Narcotics Code. For many years, all activities related to the plants and their derivatives were heavily restricted until regulatory liberalization began in February 2019 with an amendment to the Narcotics Act. Restrictions on specific types and uses of cannabis and hemp were gradually loosened, and, on June 9, 2022, Thailand became the first country in Asia to permit growing, selling, and using the plant for both medical and other permitted purposes when it delisted both cannabis and hemp plants and their unprocessed parts from the Narcotics Code.
This delisting has allowed the private sector to grow, possess, sell, and use locally cultivated cannabis and hemp plants without a license. However, the Narcotics Code still regulates cannabis and hemp extracts containing tetrahydrocannabinol (THC) of more than 0.2%. Making or handling a substance with THC over this limit still requires a license, with limited exceptions.
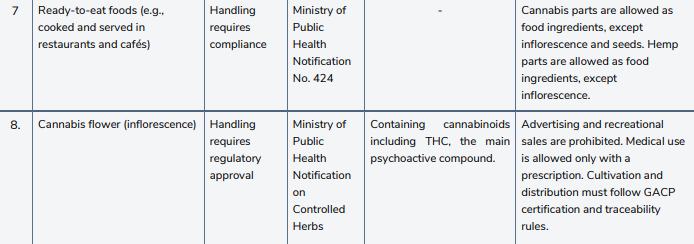
However, subsequent developments indicate that the breadth of this liberalization will likely be tempered. On June 26, 2025, The Ministry of Public Health issued the Notification on Controlled Herbs (Cannabis), legally classifying cannabis inflorescence (flowers) as a controlled herb. Cannabis flowers can still be traded. But among other new restrictions, the buyers must either have the license to sell cannabis or be individuals who have a prescription for medical cannabis from a healthcare professional. In addition, the farm as a supplier must be certified for Good Agricultural and Collection Practices (GACP). Violations may lead to up to one year in prison or a THB 20,000 (approx. USD 615) fine.
Separately, cannabis- and hemp-related products continue to be regulated by a range of productspecific legislation and overseen by various regulatory authorities.
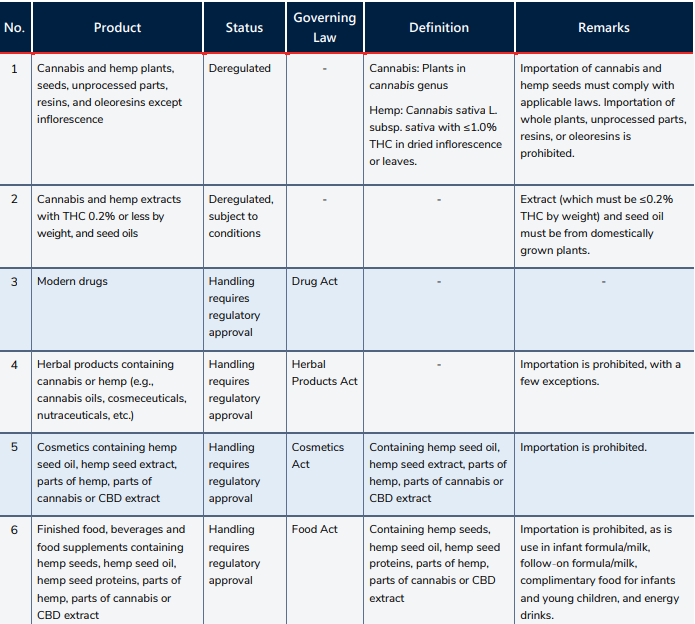
Most of the product classifications discussed here are based upon THC content, and on the presence of cannabidiol (CBD) in the products. As THC is considered a psychoactive substance— in comparison to CBD, which is not psychotropic and has been applied in various therapeutic indications—a higher THC content may therefore be associated with a greater risk of abuse, and thus stricter regulations will likely apply in Thailand. Under the most recent legal amendments, Thai law defines cannabis as in the Cannabis sativa L. subsp. indica, and hemp as Cannabis sativa L. subsp. sativa with no more than 1.0% THC by dry weight in its leaves and inflorescence.
The key regulatory body for cannabis and hemp-related products is the Thai Food and Drug Administration (Thai FDA), a government agency operating under supervision of the Ministry of Public Health. Working closely with the Narcotics Control Committee, the Thai FDA is mainly responsible for granting and administering licenses and post-marketing control, among others. Representatives of the Thai FDA also sit on most of the national policymaking committees. One of the other Thai government bodies involved in the regulation and control of cannabis and hemp is the Department of Agriculture (under the Ministry of Agriculture and Cooperatives), which administers the rules on the importation of plant seeds (e.g., cannabis and hemp seeds).
Product Classification
Activity-Focused Regulatory Overviews
Cultivating Cannabis/Hemp
Following their June 9, 2022, delisting, Thai nationals may freely cultivate cannabis and hemp plants and trade unprocessed plant parts and crude resins in Thailand without a license. Any person cultivating hemp must, however, record this online via the Thai FDA's PlookGanja application.
However, this broad liberalization is temporary, as the forthcoming Cannabis and Hemp Act is expected to impose stricter controls and a new licensing regime. Effective June 26, 2025, cannabis flowers intended for sale or export must come from cultivation sites certified under Good Agricultural and Collection Practices (GACP). Certification, conducted by DTAM inspectors, covers soil, water, pesticide use, worker hygiene, and post-harvest handling, and remains valid for one to three years with annual surveillance. Growers must maintain seed-to-sale records, including seed provenance, fertilizer and pest management logs, harvest dates, and quality-control test records. Absence of GACP certification may result in suspension of downstream dispensary and export licenses.
Companies and importation activities are subject to various regulations. For companies, agricultural activities (including growing cannabis/hemp) are still reserved for majority Thai-owned companies. Importation of cannabis/hemp plants and most unprocessed parts is still prohibited, except for cannabis/hemp seeds, which may be imported under the rules and procedures of the Plant Act (1975) and Plant Quarantine Act (1964).
Producing or Importing Cannabis/Hemp Extracts and Seed Oils
Industrial extraction of cannabis or hemp still requires a Thai FDA license under the Narcotics Code.
Since the June 9, 2022, delisting, the following products are now deregulated:
- Extracts containing 0.2% or less THC by weight produced from domestically grown plants and under license from the Thai FDA, and
- Seed oils produced from domestically grown seeds.
These deregulated products may be sold, possessed and consumed without a license under the Narcotics Code. However, handling any extract or oil that does not fall within the conditions set out above requires a license from the Thai FDA under the Narcotics Code.
Importation. Importation of any extract or oil is still prohibited.
Producing or Importing Medical Cannabis/Hemp and Other Herbal Products
Production. Irrespective of THC content, medical cannabis/hemp (including Thai traditional medicines and cannabis oils) and other herbal products (including herbal cosmeceuticals and nutraceuticals) now fall under the purview of the Herbal Product Act (2019). The act, along with its subsequent implementing regulations, provides licensing and product registration requirements for domestic production and distribution of these products.
Importation. Importation of these products is still largely prohibited with a few exceptions: Thai government agencies importing goods for treatment of patients, public academic institutions importing for research purposes, and anyone importing for human clinical trials, subject to the licensing procedures of the Thai FDA under the Herbal Products Act.
Producing or Importing Modern Drugs Formulated with Cannabinoids
Modern drugs formulated with cannabis, hemp extract, or other cannabinoids require regulatory review and licensing from the Thai FDA under the Drug Act. The licensing rules and procedures are already in place through existing ministerial regulations and notifications. Notably, a drug manufacturer or importer license is required for the business operator, and a drug registration certificate is required to market a particular modern drug formulation.
Producing or Importing Cosmetics with Hemp- and Cannabis-based Ingredients
Production. Domestically produced cosmetics may contain any or combination of hemp seed oils, hemp seed extracts, parts of hemp (except inflorescence), parts of cannabis (except inflorescence and seeds), and CBD extracts, provided that the raw materials are obtained from plants grown in Thailand. The production requires a Thai FDA license under the Cosmetics Act. Additionally, the products themselves must comply with the specifications prescribed by the relevant ministerial notifications.
Cannabis seeds, cannabis seed oils, and inflorescences of cannabis and hemp, regardless of their origins, are prohibited from cosmetic product formulations.
Importation. Importation of cosmetic products containing hemp- or cannabis-based ingredients is still prohibited.
Producing or Importing Food, Beverages, Food Supplements, or Food Supplements with Hemp- and Cannabis-based Ingredients
Production. Food products may contain hemp seeds, hemp seed oils, hemp seed proteins, parts of hemp (except inflorescence), parts of cannabis (except inflorescence and seeds), and CBD extracts, provided that these are obtained from plants grown in Thailand. For domestically produced goods, a Thai FDA license is required under the Food Act, and the product must comply with the relevant ministerial notifications.
While this allowance is applicable to many food products, there are exceptions. For example, no part of cannabis or hemp may be added to food for infants, food for young children, or energy drinks. In addition, certain foods do not allow addition of cannabis and hemp. Certain other foods allow use with conditions. Because of this variation in rules for different kinds of food, it is important to check the relevant regulations to ensure compliance.
Cannabis seeds, cannabis seed oils, and inflorescences of cannabis and hemp are not allowed as ingredients in any food product.
Importation. Importation of food products containing hemp- or cannabis-based ingredients is still prohibited—even if the cannabis- or hemp-based ingredients came from plants grown in Thailand.
To view the full article clickhere
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.




