ABSTRACT
Patents lie at the interface between technology and law. This article provides a critical review of four high profile cases from 2020 in which patents relating to pharmaceuticals were litigated in the UK Courts. The selected cases in this review involved 'sufficiency', 'novelty', 'inventiveness', 'plausibility' and 'infringement' issues. The first case is a dispute between Regeneron Pharmaceuticals and Kymab Ltd in relation to Regeneron's patent for a transgenic mouse platform. The second case relates to Pfizer's patent for their Prevnar®13 pneumococcal vaccine and the alleged infringement of this patent by Merck Sharp & Dohme. The third case concerns the validity of a patent belonging to Neurim Pharmaceuticals Ltd relating to a slow-release formulation of melatonin for treating primary insomnia. The final case is a dispute between FibroGen Inc. and Akebia Therapeutics concerning FibroGen's patents for hypoxia-inducible factor prolyl hydroxylase enzyme inhibitors ("HIF-PHIs") for use in treating anaemia. The article aims to focus on the technology behind the patents and to provide an insight into how science interacts with law in the context of patent enforcement and infringement.
INTRODUCTION
Patents sit at a point at which science and technology overlap with the law. While it is a requirement that attorneys, solicitors and judges working in patents all have a strong grasp of the technology in the sectors in which they work, quite often scientific researchers in these sectors are not exposed to patents at all, or their exposure is limited to the early stages of the life of a patent as inventors helping to prepare patent applications and provide input during prosecution of the applications to grant. Researchers will only very rarely, if ever, be involved in patent litigation.
The following is a review of a selection of cases from 2020 in which patents relating to pharmaceuticals were litigated in the UK courts. The authors of this review hope to provide researchers in the pharmaceutical fields with an insight into how science interacts with the law during patent enforcement.
The authors do not intent the review to provide an indepth analysis of the legal points in the issues but rather intend to focus on the technology involved and to identify how the basic principles of patentability and infringement were applied in the context of the issues at hand.
REGENERON PHARMACEUTICALS INC V KYMAB LTD [2020] UKSC 27
In the early 2000's, it was widely recognised that antibodies (immunoglobulins) developed using mouse (murine) platforms could be used to treat human disease. Antibodies are made by B cells and contain four polypeptide chains consisting of two identical "heavy" chains and two identical "light" chains to form the characteristic antibody "Y" structure (Fig. 1).
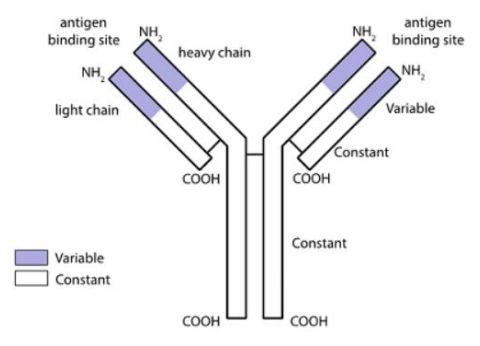
Fig. 1. Top: Schematic of an antibody structure, bottom: Regeneron Pharmaceuticals Inc (respondent) and Kymab Ltd (appellant).
Regions within these chains are known as constant (C), variable (V), diversity (D) and joining (J) as shown in Fig. 2. The heavy chain of an antibody has V, D, J and C segments whilst the light chains have only V, J and C segments. These segments are encoded in the immunoglobulin gene loci and undergo recombination during B cell maturation to produce unique antibodies for targeting specific antigens (Fig. 2).
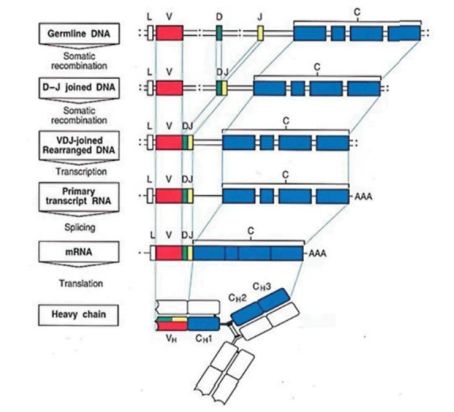
Fig. 2. Schematic of the process or rearrangement, and then transcription and translation of the heavy chain of an antibody.
Antibody therapies which had been approved for therapeutical use at this point (e.g., murine antibodies, chimeric antibodies and humanised antibodies) were known to cause adverse side effects due to the human anti-mouse antibody response (HAMA response).
Fully human antibodies produced in transgenic mice are less likely to be rejected by a patient's immune system and are more desirable, however said transgenic mice are required to have a suppressed immune response, rendering them immunologically sick and less suitable for antibody development.
The dispute concerned two European patents filed by Regeneron Pharmaceuticals, Inc. in 2002 which described genetically engineered mice having a hybrid gene structure called the "reverse chimeric locus" (Murphy et al., 2002a; Murphy et al., 2002b). This is where mouse variable immunoglobulin gene segments are replaced with human variable immunoglobulin gene segments, whilst maintaining mouse constant region segments. Such mice produce antibodies which differed from other chimeric antibodies known at the time (e.g., antibodies with mouse variable regions and human constant regions). Maintaining the mouse constant regions was found to allow for better compatibility of the antibodies with the mouse immune system and improved the immune response, near to that of wild type mice. In subsequent steps, B cells producing the desired antibodies could be removed and be genetically altered to replace the mouse regions with human regions, thus producing fully human antibodies for use in therapy. The inventive concept is best described in the following claim which was at the heart of the proceedings (Claim 1 from Murphy et al., 2002b):
"A transgenic mouse that produces hybrid antibodies containing human variable regions and mouse constant regions, wherein said mouse comprises an in situ replacement of mouse VDJ regions with human VDJ regions at a murine chromosomal immunoglobulin heavy chain locus and an in situ replacement of mouse VJ regions with human VJ regions at a murine chromosomal immunoglobulin light chain locus."
The dispute occurred when Regeneron alleged infringement of its patents by Kymab Ltd, who had developed a similar transgenic mouse platform called "Kymouse". Although infringement was found (UK 2016), Kymab counterclaimed that Regeneron's patents were invalid for being insufficiently disclosed (Fig. 3).
Sufficiency can be described as a bargain between a patentee and the public, whereby the patentee obtains a monopoly for a new invention in exchange for disclosing the invention such that it can be worked by the skilled person, thus allowing others to build upon it. Sufficient disclosure is a requirement under both the European Patent Convention and UK law (EPC 2000; UK 1977a).
To read the full article, please click here.
Originally published by British Journal of Pharmacy.
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.



