Brace for impact... These are the words we used in our 2019 article (here) for describing the European Union's ("EU") complaint against Turkey's pharmaceutical regulations. Three years have passed, and the case progressed before the World Trade Organization ("WTO"). As the case will have a significant impact on the future of Turkey's pharmaceutical industry, we wanted to give an update on the current state of the process.
But before that, let's make a quick recap of the case...
EU's complaint against Turkey
On 2 April 2019, the EU initiated a formal dispute before the WTO against Turkey's program to localize the production of pharmaceutical products.

The consultation request focuses on the measures adopted by the Turkish government in 2016 to promote the local production of pharmaceutical products, which are otherwise imported. The EU argues that localization measures discriminate against imported products and violate Turkey's obligations as a WTO member. Below, you may find a summary of the localization measures described in the EU's complaint 1:
- Localization Requirement: Turkey requires foreign producers to localize their production and provide a commitment in this regard. If such commitments are not fulfilled, Turkey excludes the relevant products from the Social Securities Institution's ("SSI") reimbursement scheme. In Turkey, majority of pharma sales are conducted through reimbursements, and products excluded from the SSI's system are effectively pushed out of the Turkish market.
- Import Restriction on Localised Products: Once the production is localized or there are locally produced alternatives of a certain product, Turkey refrains from importing equivalents of that product from producers located abroad. This restricts the international trade of pharmaceuticals and further motivates companies to localize in Turkey.
- Prioritization Measure: This claim is another extension of the localization requirement, where Turkey prioritizes applications of localized products to be accepted into the reimbursement system as well as pricing and licensing processes.
What did the WTO say?
WTO established a panel to assess the EU's complaints and Turkey's respective argumentation. Accordingly, the Panel ruled that localization and prioritization measures are discriminatory and incompatible with Turkey's commitments under WTO agreements. When we assess the case report, we see that Turkey provided the following arguments and justifications 2:
- The EU failed to establish the "existence" and "precise content" of the localization requirement as a single and cohesive measure;
- The challenged measure involves a governmental "purchase" of products, which is covered by the "government procurement" derogation in Article III:8(a) of the GATT 1994;
- Localization requirement is designed to ensure uninterrupted access to safe, effective, and affordable medicines for the Turkish population, and thus falls within the general exception in Article XX(b) of the GATT 1994 to protect human life and health;
- Localization requirement is a measure required to fulfill Turkey's obligation to ensure accessible, effective, and financially sustainable healthcare within the meaning of Article XX(d) of the GATT 1994;

Consequently, the Panel rejected Turkey's arguments and decided that the localization measure is inconsistent with Article III:4 of the GATT 1994. The Panel also rejected Turkey's argument that the EU failed to establish the existence of an overarching "prioritization measure" as defined in the complaint 3.
What's next?
On 25 April, Turkey initiated an appeal process against the Panel report. In the appeal, Turkey will try to reverse the findings, conclusions, and recommendations of the Panel. The appeal review will focus on the errors of law and legal interpretation contained in the Panel Report.
While the next steps seem to be laid out in the WTO procedures, there is another twist in the plot. In 2019, the United States blocked appointments to the Appellate Body over concerns about the body's independence and activism. This triggered a chain reaction and severely restricted the functionality of WTO's Dispute Settlement System. Accordingly, the parties needed to take an alternative approach to consummate the appeal review.
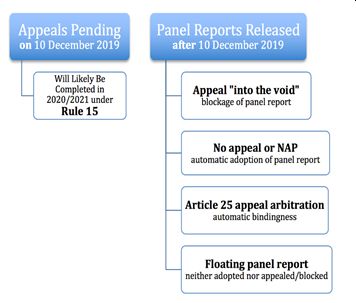
This is where the long-forgotten "Article 25" takes the center stage. This provision provides a parallel dispute settlement structure within the WTO framework. Under Article 25 of the Understanding on Rules and Procedures Governing the Settlement of Disputes ("DSU"), the parties are allowed to circumvent the ongoing gridlock and avert the imminent collapse of the WTO's dispute settlement system 4. Parties may initiate this arbitration, only if the Appellate Body is not able to hear an appeal in this dispute (Article 16.4 and 17 of the DSU). This procedure has only been used once before in the body's 27-year history 5.
That said, as Panel reports keep piling up and the Dispute Settlement System is incapable of processing the pending cases, we may see more arbitrations under Article 25 in the future 6. If the appeal process results in the EU's favor, Turkey will need to revise the localization measures and bring its pharma policies in conformity with the WTO agreements. In such a case, localization measures will be removed within a period negotiated with the EU or fixed by a WTO arbitrator 7.
Footnotes
2. Panel Report p. 61;90
4. https://www.csis.org/analysis/article-25-effective-way-avert-wto-crisis
5. Turkey uses rare clause to appeal EU pharmaceuticals case at WTO | Reuters
6. Please see the infographic by International Economic Law and Policy Blog for additional information on alternative scenarios for WTO dispute settlement: https://ielp.worldtradelaw.net/2019/07/scenarios-for-wto-dispute-settlement-post-2019.html
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.



