- within Food, Drugs, Healthcare and Life Sciences topic(s)
- with readers working within the Healthcare industries
- within Food, Drugs, Healthcare and Life Sciences topic(s)
- within Finance and Banking, Tax and Immigration topic(s)
Software as a Medical Device (SaMD) is becoming increasingly essential in modern healthcare. A Software as a Medical Device simply refers to any software intended for medical purposes without being part of a physical medical device and can perform medical functions independently such as diagnose, treat, mitigate, or prevent diseases. SaMDs may include in-vitro diagnostic (IVD) medical devices; software that runs on general-purpose computing platforms; and software that functions as a module within another medical product. Also, mobile applications that meet these criteria are also classified as SaMD.
In order to ensure public health, safety, and effectiveness, all SaMD products intended for use in Nigeria, whether manufactured or imported, must be registered with the National Agency for Food and Drug Administration and Control (NAFDAC).
In this article, we provide a clear, step-by-step guide for importers and manufacturers on the registration process of software as a medical device.
Categorization of Software as a Medical Device (SaMD)
The categorization of Software as a Medical Device (SaMD) is based on its definition statement and its impact on healthcare decisions and patient outcomes. For example, software that helps diagnose a life-threatening disease (like cancer) will be in a higher category than an app that tracks daily fitness levels.
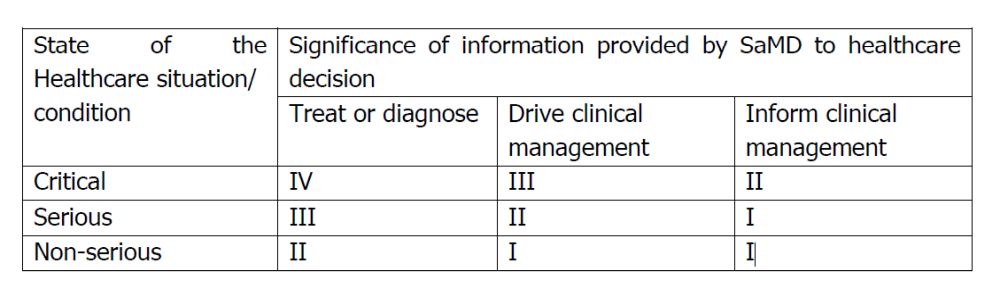
There are four categories of SaMDs based on the levels of impact on the patient or public health where accurate information provided by the SaMD to treat or diagnose, drive or inform clinical management is vital to avoid death, long-term disability or other serious deterioration of health, mitigating public health. Even when integrated with other devices or systems, each SaMD is categorized independently based on its own definition statement.
The four categories (I to IV) reflect the level of risk, with Category IV having the highest impact on health and public safety. If a SaMD is used for multiple conditions, it is classified at the highest relevant category; while Category I has the lowest. A calorie counter or fitness tracker app. For example, an app that reminds users to take medication or a software that logs blood pressure readings without making treatment recommendations are considered low risk SaMD; a dermatology app that classifies skin conditions for review by a healthcare provider will be considered moderate or medium risk SaMD; and a radiology imaging analysis tool that assists in detecting tumors is considered high risk SaMD.
Furthermore, any changes to the SaMD that affect its function, will require a reassessment of its category.
Below is a summary of the categorization at a glance.
To view the article in full click here
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.


