Holding
In Acadia Pharms., Inc. v. Aurobindo Pharma Ltd., No. 20-985-GBW (D. Del. Dec. 2023), the district court granted Acadia's motion for summary judgment of no invalidity for obviousness-type double patenting. The asserted patent was not a proper obviousness-type double patenting reference and the safe harbor of 35 U.S.C. §121 applied to protect the original application.
Background
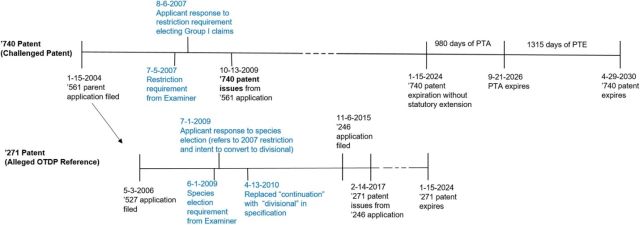
Defendants filed an ANDA for a generic version of Nuplazid®. The FDA tentatively approved the ANDA. In the infringement lawsuit, the parties agreed to limit the dispute to a validity determination of claim 26 of U.S. Pat. No. 7,601,740 ("the '740 patent"). According to the Defendants, claim 26 was invalid in light of claim 5 of U.S. Pat. No. 9,566,271 ("the '271 patent").
Claim 26 of the '740 patent recites a tartrate salt of pimavanserin.
Claim 5 of the '271 patent is directed to a method for treating hallucinations by administering a tartrate salt of pimavanserin.
Timeline from the district court decision (Exhibit A from Acadia's Answering Brief (D.I. 261):
District Court
Section 121 protects both patents issuing from original applications in which a restriction requirement has been made and patents issuing on applications filed as a result of a restriction requirement. Therefore, "filed before the issuance of the patent" applies to a challenged divisional patent, not a challenged original application.
Since the reference patent, the '740 patent, was "filed as a result of" a restriction requirement, the safe harbor applied to protect the '271 patent. Id. The court agreed that "an application is 'filed as a result of' the restriction requirement when an initially non-divisional application is amended to comport with a restriction requirement in another patent." Id. See Boehringer Ingelheim Intern. GmbH v. Barr Labs., Inc., 592 F.3d 1340, 1353-54 (Fed. Cir. 2010).
The '271 patent claims are entitled to their full term (including PTA) as the earlier-filed, earlier issued patent. Patents and applications must be later-filed to be obviousness-type double patenting references. Id. at *14. As a later-filed patent, the '271 patent is not a proper obviousness-type double patenting reference against the earlier-filed '740 patent.
Note that the court found that Cellect did not apply because here the issue was a threshold one as to the availability of a reference while in Cellect, the availability of the reference was not argued. Instead, the parties disputed whether OTDP could cut off PTA and then, "despite its clear statement that OTDP only applies to 'later-filed obvious variations of earlier-filed, commonly owned claims,' [the court] invalidated an earlier-filed patent. Id. at * 22. The court in Acadia recognized that "another court in this district has interpreted Cellect to cut off OTDP even for first-filed, first-issued patents. Allergan USA, Inc. v. MSN Labs. Private Ltd. et al., C.A. No. 19-1727-RGA, 2023 U.S. Dist. LEXIS 135710." Id. at *23, FN 4. But the court again distinguished the issue in dispute; "the Allergan Court did not analyze the language in Cellect discussing 'later-filed obvious variations of earlier-filed, commonly-owned claims.' .... Accordingly, the Court does not find itself persuaded by Allergan." Id.
Takeaways
Prosecution counsel is reminded that amending a CON to be a DIV is allowed to obtain safe harbor protection under § 121 (as long as consonance is maintained).
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.


