Holding
Petitioner Dr. Reddy's Laboratories challenged the patentability of several claims of U.S. Patent No. 11,110,087 ("the '087 patent") on 35 U.S.C. § 102 anticipation grounds. IPR2023-00052, Paper 7 at *4. Even though this petition was for an inter partes review ("IPR") proceeding, the Patent Trial and Appeal Board ("the Board") analyzed written description under 35 U.S.C. § 112(a) to assess whether the '087 patent should receive the benefit of priority to the original application. The Board instituted the IPR, finding that there was a reasonable likelihood that most of the challenged claims of the '087 patent "are not entitled to a benefit of priority prior to the November 2018 Venclexta label" and are therefore anticipated. Id. at *37.
Background
The '087 patent is directed to a combination therapy with a dosage schedule. Id. at *3. Of the challenged claims, claims 1 and 19 are independent:
1. A method for treating lymphoma or leukemia in a human patient, the method comprising orally administering once daily GDC-0199 to the patient in dosing cycles comprising escalating doses, wherein the escalating doses comprise a dose of 100 mg of GDC-0199 per day, followed by orally administering to the patient a dose of 200 mg of GDC-0199 per day, followed by orally administering to the patient a dose of 400 mg of GDC-0199 per day.
19. A method for treating lymphoma or leukemia in a human patient, the method comprising orally administrating once daily GDC-0199 in escalating doses to the patient, wherein the escalating doses comprise a daily dose of 100 mg of GDC-0199.
PGR v. IPR
The Board rejected Patent Owner's argument that that this IPR petition with its §112 arguments was "an improper attempt to circumvent the statutory deadline to bring a post-grant review challenge." Id. at *8. Patent Owner provided no basis for the position that a petitioner is required to file a PGR petition when a patent is eligible instead of waiting and filing an IPR. Id. at *11.
Anticipation, Priority & Written Description
Petitioner contended that the challenged claims were anticipated by the label on Venclexta (a.k.a. GDC-0199) and published as of November 2018. Id. at *4.
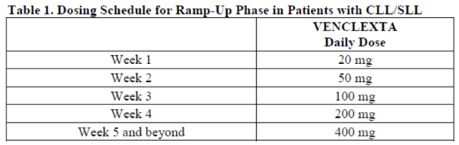
Table 1. Dosing schedule on the Venclexta label.
The anticipation challenge was "predicated on whether the claims at issue receive the benefit of priority to the original Specification" and this in turn required consideration of whether the "challenged claims find adequate written description support in the priority document." Id. at *13. Petitioner contended that the original specification did not provide adequate guidance to choose from "various laundry-listed elements to reach the claimed dosing regimens" and that the specification did not describe a GDC-0199 monotherapy. Id. at *13-14.
The Board rejected Patent Owner's defense that § 112 written description issues cannot be raised in an IPR. "Where, as with Ground 1, the effective filing date of a claim is an issue in a § 102 or § 103 analysis, courts have considered whether the priority document provides descriptive support for the claim." Id. at *13. The Board considered this approach to be "consistent with Indivior [UK Limited v. Dr. Reddy's Labs. S.A., 18 F.4th 1323 (Fed. Cir. 2021)]." Id. at *11. However, the Board agreed with Patent Owner that Petitioner's enablement argument "is not an allowable ground in an inter partes review proceeding." Id. at *12. By filing an IPR petition instead of a PGR petition, "Petitioner chose to limit the issues to grounds under § 102 and § 103." Id. at *13.
The Board decided for institution purposes that there is a reasonable likelihood that claim 1 and its dependent claims 2-4, 8, 10-14 and 18 lacked written description support in the earlier specifications and are not entitled to the benefit of priority to antedate the Venclexta label. Id. at *22. Claim 1 lists a dosing regimen that is purportedly not found in a single example but is instead sourced from disparate parts of the specification, with lists of potential cancers to treat with the dosing regimen and lists of "different dosing cycle lengths and treatment phases." Id. at *22-23.
For claims 20-26, the Board likewise preliminarily found that the specification lacked written description support. Id. at *31. Claims 20-26 depend from claim 19 and recite additional dosing schemes that the Board stated involved "selection from a multitude of dose parameters" in the specification without an indication of which doses were therapeutically effective. Id. Claim 25 recites continuing the dosing schemes until disease progression, but the Board indicated that there was a "lack of guidance on 'disease progress'" and that the specification merely rendered the invention of claim 25 obvious. Id. at *31-32.
However, the Board found that the same reasoning did not apply to claim 19. Id. at *27. Figure 2 of the '087 specification provided literal written description support for claim 19 by teaching a particular exemplary dosing scheme. Id. at *28. The Board found that Petitioner had not successfully shown that there was a reasonable likelihood that independent claim 19 lacked written description support. Id. at *31.

Figure 2. Dosing schedule administering GDC-0199 in escalating doses for 3 weeks prior to antibody administration.
Take-Aways
Written description under 35 U.S.C. § 112(a) may need to be considered to determine whether the claims at issue receive the benefit of a claimed priority. However, § 112 enablement grounds cannot be raised in an IPR proceeding.
The failure to petition for PGR is not a basis for denial in an IPR. Section 311(a) does not restrict eligible petitioners to those who could not have filed a post-grant review.
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.

