U.S. Food and Drug Administration (FDA) Commissioner, Dr. Scott Gottlieb, has made generic drugs and drug pricing an agency priority by emphasizing the critical value of generic drugs to public health. In 2017, 1027 total generic drugs were approved, which was the highest number of generic drugs approved in a single year.1 Table 1 below illustrates 2017 approvals by month.
Table 1: 2017 Generic Drug Approvals

The Office of Generic Drugs (OGD) released its 2017 Annual Report last month. A copy of the 2017 Annual Report can be obtained here. OGD ensures, through a scientific and regulatory process, that Americans receive safe, effective, and high-quality generic drugs. FDA-approved generic drugs account for more than 88 percent of prescriptions filled in the United States. All approved generic drugs have the same high quality, strength, purity, and stability as brand-name drugs. The generic manufacturing, packaging, and testing sites must pass the same quality standards as those of brand name drugs. OGD is comprised of an immediate office and four subordinate offices, totaling approximately 450 employees. Among other things, OGD provides the following critical functions:
- Reviews applications for the approval of generic drugs (e., Abbreviated New Drug Applications);
- Serves as the central point of contact between applicants and the FDA Generic Drug Program; and
- Provides guidance and regulatory oversight to industry on a variety of clinical, scientific, and regulatory matters relating to generic drugs.
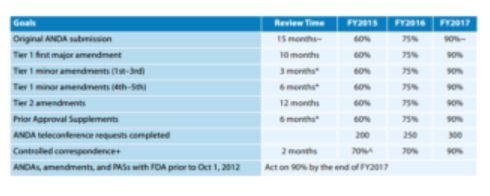
In 2017, the Generic Drug User Fee Amendments (GDUFA) were reauthorized. The GDUFA performance goals include time frames within which FDA has commits to take a first action on an abbreviated new drug application (ANDA), an amendment to an ANDA, and prior approval supplements, which are post-approval changes requiring a supplemental submission and approval. Table 2 below highlights some of major accomplishments of the FDA under the GDUFA performance goals and commitments.
Table 2: Major GDUFA Performance Goals and Commitments
In 2017, OGD issued 178 new product-specific guidances for industry. OGD develops product-specific guidances soon after brand-name drugs are approved to facilitate the efficient premarket development, successful filing, and substantive review of generic drug products. The recommendations in the guidances describe the FDA's current thinking and expectations on the development of a specific drug and consider the unique features of the reference listed drug that should be incorporated into a generic version of that drug. OGD also develops and issues product-specific guidances based on requests from industry and public health priorities. An OGD working group reviews requests for product-specific guidances and makes recommendations to industry to meet current and anticipated patient and industry needs.
FDA Commissioner Gottlieb introduced the Drug Competition Action Plan (DCAP), which focuses on generic competition, the critical value of generic drugs to public health, and concerns over the increasing cost of medicines to consumers. Among other things, DCAP is intended to reduce the price consumers pay for medicines and to balance the goals of the Hatch-Waxman Amendments to incentivize new drug innovation and facilitate the generic drug access that Congress intended.
Medicines become more affordable through competition when multiple generic versions of brand-name drugs are available to patients. Considered a public health priority, FDA prioritizes reviews of applications for first generics, which open the market to generic competition. DCAP recognizes the market value in having three generics approved and prioritizes OGD's review of applications up to the third approval. OGD approved 80 first generic drugs in 2017. Table 3 below identifies several illustrative first approved generic drugs:
Table 3: Illustrative 2017 First Generic Drug Approvals

In fruther response to DCAP, OGD took several steps to increase competition for prescription drugs and facilitate entry of low-cost alternatives to the market including:
- Publishing a list of off-patent, off-exclusivity branded drugs (based on drug products) without approved generics to improve transparency and encourage the development and submission of ANDA's in markets with limited competition;
- Expediting the review of a greater numbers of generic drug applications when competition is limited (e., to prioritize review of generic drug applications until there are three generics approved for a brand product);
- Enhancing development and review of ANDAs for complex generic drug products, which can require more review cycles, through the publication of the draft guidances for industry;2
- Reviewing our Hatch-Waxman implementation and soliciting input on places where FDA's regulations are being used in ways that may create obstacles to generic access, instead of ensuring the vigorous competition Congress intended.
Finally, the Annual Report, provides a brief summary of OGD's GDUFA regulatory science research tools for industry to develop new generic drug products and for FDA to evaluate the equivalence of a proposed generic drug as well as significant research accomplishments.
Footnotes
1 The 1,027 total generic approvals included 843 full approvals and 184 tentative approvals.
2 OGD held three public meetings in October 2017 and issued 47 product-specific guidances on the development of complex generic drug products.
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.


