In science there is only physics; all the rest is stamp collecting1.
Results! Why, man I have gotten a lot of results. I know several thousand things that won’t work2.
We have learned from experience that truth will out. Other experimenters will repeat your experiment and find out whether you were wrong or right. Nature’s phenomena will agree or they will disagree with your theory. And although you may gain some temporary fame and excitement, you will not gain a good reputation as a scientist if you haven’t tried to be very careful in this kind of work. And it’s this type of integrity, this kind of care not to fool yourself that is missing to a large extent in much of the research in cargo cult science3.
Introduction
This discussion is with particular, but not exclusive, reference to practice in the pharmaceutical industry for claiming new chemical entities, and in particular to the feasibility of defining a genus of compounds that validly covers compounds that have not yet been made and tested.
In the early days of the pharmaceutical industry claims were to single compounds. The well-known Felix Hoffmann US patent No 644077 granted in 1900 for aspirin provides an example. Another researcher had previously described acetylsalicylic acid, but Hoffmann demonstrated that the compound that the earlier researcher had made did not possess the correct properties. Hoffmann had developed a new process that gave "the real acetyl salicylic acid" which "exhibits therapeutical properties" and claimed:
"As a new article of manufacture the acetylsalicylic acid having the formula

being when crystallised from dry chloroform in the shape of white glittering needles, easily soluble in benzene, alcohol and glacial acetic acid, difficultly soluble in cold water, being split by hot water into acetic acid and salicylic acid, melting at about 135°C, substantially as hereinbefore described."
Compounds isolated from nature and produced synthetically could also be claimed. An example is provided by US patent 3880888 (Firmenich S.A.) which reports the isolation of the compound 3,4,7-trimethyl-2-oxo-1,6-dioxa-spiro[4.5]dec-3-ene from an essential oil derived by distillation of Burley tobacco. The compound is said to provide a mild herbal or woody note to the tobacco, and is claimed as a composition of matter consisting essentially of a compound of the formula

As is well known, the pharmaceutical industry was an offshoot from the dyestuff industry. From the beginning of the 20th century onwards, the practice developed within that industry of claiming classes of dyestuffs generically by means of structural formulae. US-A-1744172 provides an example of so-called Markush practice as it had developed by the mid 1920’s. The patentees had claimed a novel class of azo dyes which had the benefit of producing durable colours on cotton and other vegetable fibres, and which are defined in claim 2 of the patent (with slight editing) as follows:
"As new products azo dyestuffs having most probably the general formula:

wherein Y stands for alkyl, oxyalkyl, oxyaryl or halogen; X represents either the sulfone group SO2 or the carbonyl group CO, and R1 represents ... alkyl, aryl, aralkyl ... which are in the dry state reddish to dark coloured powders, soluble in concentrated sulphuric acid with from blue to dark violet colours ... and which when produced on vegetable fibres yield reddish shades of excellent fastness to kier boiling."
The patentability of the compounds of this genus flows from the link between the chemical structure and the newly discovered and valuable properties possessed by the compounds of that structure. That formulation is consistent with the question put forward by Sir Stafford Cripps K.C. in UK proceedings between Sharp & Doehme v Boots4 which concerned the manufacture of certain alkyl-substituted resorcinols which were known as antiseptics (at that time chemical product claims were not allowed in the UK). Published papers had already disclosed that the C1-C4 members of the class had been made and that antiseptic activity increased as the number of carbon atoms in the alkyl chain increased. The patentees averred that because drug research was empirical, the skilled person knew in advance neither that the higher alkyl resorcinols could be produced by the claimed method nor what their antiseptic properties would be. The compound that proved to be of therapeutic value was n-hexyl resorcinol which is of formula:

The defendants replied that because therapeutic activity increased in the known compounds from C1 to C4, it was obvious to make the higher members of the series. Sir Stafford Cripps argued that the question concerning inventive step that the court had to decide was based on whether there was an obvious link between structure and value, and was as follows:
"Was it for practical purposes obvious
- to any skilled chemist
- in the state of chemical knowledge existing at the date of the patent, which consisted of
- the chemical literature available, a selection of which appeared in the Particulars of Objections, and
- his general chemical knowledge,
- that he could make valuable therapeutic agents by making the higher alkyl resorcinols?" (Paragraphing and emphasis added).
The same link between structure and value is implicit in the requirement for utility in US law, as is apparent, for example, from the following passage from Brenner v Mason which calls for the product to be useful, i.e. to have a "specific benefit":
"Until the process claim has been reduced to production of a product shown to be useful, the metes and bounds of that monopoly are not capable of precise delineation. It may engross a vast, unknown, and perhaps unknowable area. Such a patent may confer power to block off whole areas of scientific development, without compensating benefit to the public. The basic quid pro quo contemplated by the Constitution and the Congress for granting a patent monopoly is the benefit derived by the public from an invention with substantial utility. Unless and until a process is refined and developed to this point - where specific benefit exists in currently available form - there is insufficient justification for permitting an applicant to engross what may prove to be a broad field."
In the azo dyestuff case referred to above, it is clearly credible that the whole class of compounds would dye cotton with the indicated combination of colour and resistance to boiling. However, the credibility of similar Markush claims for pharmaceuticals or agrochemicals is open to question since human, animal and living plant cells are more complex than cotton fibres.
Generic claims to the results of empirical research
Finding pharmaceutically active compounds has been at least until recently a task of empirical research of the kind pioneered by Thomas Alva Edison, and the rules applicable to patents of this category have been known at least since the decision of the US Supreme Court in the Incandescent Lamp case which concerned a third party patent which was alleged to be infringed by the electric lamps being made and sold by Edison licensees. The third party patent purported to monopolise all carbonised fibrous or textile materials for use as filaments for electric lamps, as is apparent from the following main claim:
"An incandescing conductor for an electric lamp, of carbonized fibrous or textile material, and of an arch or horseshoe shape, substantially as hereinbefore set forth."
The evidence adduced in the case showed that the researches carried out by the third party could not support such a broad monopoly and that the field was unpredictable. The factual background and the reasoning of the court will be apparent from the following passages from the Opinion:
"Is the complainant entitled to a monopoly of all fibrous and textile materials for incandescent conductors? If the patentees had discovered in fibrous and textile substances a quality common to them all, or to them generally, as distinguishing them from other materials, such as minerals, etc., and such quality or characteristic adapted them peculiarly to incandescent conductors, such claim might not be too broad. If, for instance, minerals or porcelains had always been used for a particular purpose, and a person should take out a patent for a similar article of wood, and woods generally were adapted to that purpose, the claim might not be too broad, though defendant used wood of a different kind from that of the patentee. But if woods generally were not adapted to the purpose, and yet the patentee had discovered a wood possessing certain qualities, which gave it a peculiar fitness for such purpose, it would not constitute an infringement for another to discover and use a different kind of wood, which was found to contain similar or superior qualities.
The present case is an apt illustration of this principle. Sawyer and Man supposed they had discovered in carbonized paper the best material for an incandescent conductor. Instead of confining themselves to carbonized paper, as they might properly have done, and in fact did in their third claim, they made a broad claim for every fibrous or textile material, when in fact an examination of over 6,000 vegetable growths showed that none of them possessed the peculiar qualities that fitted them for that purpose. Was everybody, then, precluded by this broad claim from making further investigation? We think not.
The injustice of so holding is manifest in view of the experiments made, and continued for several months, by Mr. Edison and his assistants, among the different species of vegetable growth, for the purpose of ascertaining the one best adapted to an incandescent conductor. Of these he found suitable for his purpose only about three species of bamboo, one species of cane from the valley of the Amazon (impossible to be procured in quantities on account of the climate), and one or two species of fibers from the agave family. Of the special bamboo, the walls of which have a thickness of about 3/8 of an inch, he used only about 20/1000 of an inch in thickness. In this portion of the bamboo the fibers are more nearly parallel, the cell walls are apparently smallest, and the pithy matter between the fibers is at its minimum. It seems that carbon filaments cannot be made of wood, that is exogenous vegetable growth, because the fibers are not parallel, and the longitudinal fibers are intercepted by radial fibers. The cells composing the fibers are all so large that the resulting carbon is very porous and friable. Lamps made of this material proved of no commercial value. After trying as many as 30 or 40 different woods of exogenous growth, he gave them up as hopeless. But finally, while experimenting with a bamboo strip which formed the edge of a palm-leaf fan, cut into filaments, he obtained surprising results. After microscopic examination of the material, he dispatched a man to Japan to make arrangements for securing the bamboo in quantities....
... how would it be possible for a person to know what fibrous or textile material was adapted to the purpose of an incandescent conductor, except by the most careful and painstaking experimentation? If, as before observed, there were some general quality, running through the whole fibrous and textile kingdom, which distinguished it from every other, and gave it a peculiar fitness for the particular purpose, the man who discovered such quality might justly be entitled to a patent; but that is not the case here. An examination of materials of this class carried on for months revealed nothing that seemed to be adapted to the purpose; and even the carbonized paper and wood carbons specified in the patent, experiments with which first suggested their incorporation therein, were found to be so inferior to the bamboo, afterwards discovered by Edison, that the complainant was forced to abandon its patent in that particular, and take up with the material discovered by its rival. Under these circumstances, to hold that one who had discovered that a certain fibrous or textile material answered the required purpose should obtain the right to exclude everybody from the whole domain of fibrous and textile materials, and thereby shut out any further efforts to discover a better specimen of that class than the patentee had employed, would be an unwarranted extension of his monopoly, and operate rather to discourage than to promote invention."
The views of the US Supreme Court coincide with those of the English judges. In the case of claims to newly discovered compounds, the basic principle is as set out by the House of Lords in May & Baker v Boots7 which explains that generic claims are permissible where there is a rational basis for them, and sets out the following principles for research in an empirical art:
(i) in a field where progress is by empirical discovery, an invention must be in respect of a substance which has actually been produced, since there cannot be an empirical discovery in respect of a bare formula;
(ii) each new compound is a separate invention since its worth is a new discovery; but
(iii) where the chemist has found some law or principle by which he can predict therapeutic effect in advance so that each of a group of new products will be of value, the art will have lost its empirical nature, at least to some extent (i.e. a Markush claim may be allowable).
The factual situation in May & Baker is of interest. The invention claimed in the patent in issue GB-A-533495 covered sulfathiazole (M & B 760) and sulfamethylthiazole, the first of which was the most important of the sulfa antibacterials and is of the formula indicated below:
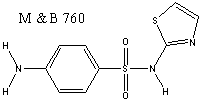
The specification did not have specific claims directed to the two exemplified active compounds. It only had generic claims to benzene sulfonamido thiazole derivatives, the genus of compounds claimed being described in words rather than by a structural formula. The description explained that the NH2 group could be substituted amino (e.g. alkylamino, N-acyl-N-alkylamino or aralkylamno) and that the benzene ring could have alkyl substituents. The thiazole ring could also be substituted. The resulting genus covered tens of millions of compounds, and although it covered compounds additional to the two compounds exemplified that had been found to be active, it admittedly also covered compounds that were inactive. The patentees therefore proposed to replace their generic claim by a claim to the two exemplified compounds, arguing that it was apparent on the face of the specification that they were drawing an inference from their specific data and should not be penalised if that inference proved unfounded. In a split (3:2) judgment the House of Lords rejected this approach and said8:
"The argument and the construction of the specification implied in it are open to grave objections. They set aside the plain categorical statements of the specification that the invention consists in the manufacture of new benzene-sulphonamido-thiazole derivatives, and that the new benzene-sulphonamido-thiazoles have especially favourable therapeutic properties. These general statements are no longer to be accepted as statements of fact, and they are to be regarded rather as a scientific hypothesis. It seems to have been forgotten that they were the basis of a patent grant just because they were treated as statements of fact, and that they were put into the specification and followed by the commensurate claims for the purpose of obtaining a monopoly in the large field covered by the claims ... Let it be here recalled that the Appellants have acquiesced in the finding of Jenkins J. that on a proper construction of the specification there is an assertion that every substance capable of being produced by the described methods from the prescribed materials was of the especially therapeutic value, and that they have obtained from the same learned judge a finding that they believed what they asserted, that is they committed themselves to this statement as a true generalization from their experimental data. Having so committed themselves they cannot now draw back and say that they are committed only to the truth of their assertions about two specific substances and to an unproved scientific hypothesis."
The generic claim was therefore invalid, and in the absence of specific claims to the two exemplified compounds, the patent was held to be unenforceable.
The same principles were followed by the UK House of Lords in Biogen v Medeva9:
"Thus if the patentee has hit upon a new product which has a beneficial effect but cannot demonstrate that there is a common principle by which that effect will be shared by other products of the same class, he will be entitled to a patent for that product but not for the class, even though some may subsequently turn out to have the same beneficial effect..... On the other hand, if he has disclosed a beneficial property which is common to the class, he will be entitled to a patent for all products of that class (assuming them to be new) even though he has not himself made more than one or two of them."
In the EPO, although technical analysis is conducted within the formal framework of problem/solution analysis, the outcome principally determined by whether the claimed subject matter leads to a new effect, some new function or advantage that cannot be predicted from the teachings of the prior art references considered collectively10. Szabo made that clear, for the chemical field at least,11 when he said that:
"The practice of the Board of Appeal (Chemistry) has been relying on an effect-centered problem and solution approach to the question of inventive step."
This observation is supported by the decision in the well-known case of Agrevo/Triazoles12, which concerned a class of herbicidal triazole sulphonamides. The dispute was whether the whole class of compounds had to herbicidal in order to establish patentability, or whether it was enough for the applicants to show that:
(a) some of the claimed compounds were herbicides; and
(b) the cited art did not teach the skilled person to make any compound within the class.
The origin of the objection was that the genus of compounds claimed included open-ended definitions that covered very large substituents and the Examining Division considered that it was not plausible that the claimed compounds had utility for all possible substituents. The applicants argued that the Board need only consider whether the skilled person would make the claimed compounds starting from the prior art teachings – i.e. the relevant question was what the public would do, not what the inventor had achieved. The Board rejected the applicant’s argument in uncompromising terms, and based its opinion on the legal principle that the extent of the patent monopoly should correspond to and be justified by the technical contribution to the art that is contained in the specification. It went on to say that mere structural ingenuity was not sufficient. If the result that the skilled person was seeking to achieve was simply obtaining further chemical compounds, then all known chemical compounds were equally suitable as starting points and all known methods of transformation might be used, so that the selection of particular compounds to be made was a mere arbitrary choice. For that reason
"…the selection of such compounds, in order to be patentable, must not be arbitrary but must be justified by a hitherto unknown technical effect which is caused by those structural features which distinguish the claimed compounds from the numerous other such compounds ... It follows directly from these considerations that a technical effect which justifies the selection of the claimed compounds must be one which can fairly assumed to be produced by substantially all the claimed compounds"
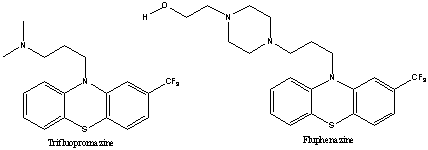
The UK decision in Olin Mathieson v Biorex13 provides an example of a generic definition in the pharmaceutical field that was not successfully challenged. The tranquiliser chlorpromazine (Largactil) had been patented in 1950 and provided a breakthrough in the treatment of mental illness. It was of formula:

The discovery of chlorpromazine stimulated structure-activity investigations aimed at the production of compounds with improved properties. The patentees had discovered inter alia the drugs trifluopromazine and fluphenazine, which were based on a ring CF3 substitution and were of formulae:
The patent in issue GB-A-857547 (which had been divided from GB-A-857546 claiming a wider genus) covered a genus of compounds of the formula:
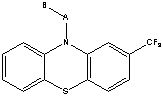
wherein A is a lower alkylene radical and B is a basic nitrogen-containing radical with fewer than twelve carbon atoms14. The compounds were said to be antihistaminic, anti-emetic and tranquilising agents and to be useful for the treatment of psychotic states, the compound trifluopromazine being disclosed as more potent than chlorpromazine. The defendants proposed to manufacture a compound called trifluoperazine (Stelazine) that was covered by the generic claims of the patent in issue but had not been exemplified or specifically disclosed. It was of formula:

An initial allegation of non-enablement was not maintained, and the argument centred on whether the disclosure supported the broad generic scope of the independent claims. The Court’s decision to uphold the generic claims was based primarily on the facts that:
(a) the side chains covered by the patent in issue had been disclosed in earlier patents concerning phenothiazines and had been shown to be active, (b) there was no reason to suppose that change of the ring substituent from Cl to CF3 would affect the range of side chains that was compatible with activity,
(c) for any given side chain the CF3 substituent in the 2-position gave the highest activity, and
(d) all the CF3-substituted phenothiazl compounds that the plaintiffs had made and tested were therapeutically active.
A further instance where the same principles were adopted arises in Chiron v Murex15 which concerned the sale of test kits for the hepatitis C retrovirus. The patentees had isolated form an infected chimpanzee called Rodney a polynucleotide that contained the instructions for making the antigenic determinant of the virus i.e. the site on the viral particle to which antibodies of the immune system attached themselves. They had then determined the sequence of that sample. These were the critical steps in identifying the cause of HCV infections and in harnessing the HCV antibody:antigen reaction to produce diagnostic test kits. The patent claimed inter alia:
"A polypeptide in substantially isolated form comprising a contiguous sequence of at least 10 amino acids encoded by the genome of Hepatitis C virus (HCV) and comprising an antigenic determinant, wherein HCV is characterised by:
(i) a positive stranded RNA genome;
(ii) said genome comprising an open reading frame (ORF) encoding a polyprotein; and
(iii) said polyprotein comprising an amino acid sequence having at least 40% homology to the 859 amino acid sequence in Figure 14."
The defendants alleged that the claim did not relate to a new class of chemicals because the members would be different chemically or biologically from one another, and because there was not prescribed a formula that linked them. The patentees replied that the link was their discovery of the virus and the presence of the antigenic determinants of the virus to which HCV antibodies would bind. The Court held that the facts differed from those in Biogen and stated:
"We prefer the submissions for Chiron. We will take claim 1 as the paradigm. There is nothing in that claim as a matter of language or in the specification as a whole to differentiate between one polypeptide falling within the claim and any of the millions of others. At the other end of the scale, there is no doubt that it was the finding and sequencing of the Rodney virus genome which was of the greatest importance for this discovery and analysis enabled the antigenic determinants to be found in the protein expressed by the sequence. In view of the fact that an antigenic determinant can only be defined by reference to the antibody which binds to it, and the further fact that the immune response of an individual produces a whole range of antibodies to any given virus it is not possible to define antigenic determinants by reference to any common chemical formula. Thus the invention is the chemical comprising at least 10 amino acids in which there is an antigenic determinant to which an antibody to HCV will bind. In our view, both in substance and in form, this is a single invention properly defined by the common denominator of the existence of an antigenic determinant of HCV, notwithstanding that the resultant polypeptides will have divergent characteristics in other respects. The invention of one is the invention of all of them because that which is common to all is of the essence of the discovery and that which distinguishes one from the other is irrelevant to that discovery."
A recently litigated Markush claim in the pharmaceutical industry – You may be in trouble if you Markush!
Monsanto et al v Merck16 concerned infringement of a patent covering non-steroidal anti-inflammatory drugs. Pumfrey J. at first instance handed down a 78-page opinion on 4th February 2000.
By way of background, US-A-3743656 (Brown) had previously disclosed a class of diaryl heterocyclic compounds said to be anti-inflammatory agents and of the formula:

in which:
the heteroatom X could be O, S or –NH-;
Z could be hydrogen;
Ar and Ar1 could be phenyl or substituted phenyl in which the substituents could be F or CH3SO2; and
R represented an alkyl group.
Amongst the specific disclosures in the Brown specification was a sub-class of compounds of the formula:
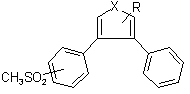
in which X and R have the meanings given above.
DuPont had been working on diaryl heterocyclic compounds from 1979/80 onwards, and had published in 1989 details of a compound called DuP 697 that was said to combine good anti-inflammatory characteristics with low gastric irritancy.
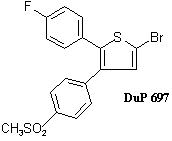
The properties of that compound had been described in a slide shown at a prostaglandin conference as follows:
- Active as an anti-inflammatory, antipyretic and in models of inflammatory pain.
- Selective inhibition of cyclooxegenase (COX) in cellular and enzymatic assays.
- Lack of platelet activity and safety profile may reflect in vivo results of selective CO inhibition.
- May be a useful tool to understand role of COX 1 and COX 2.
Despite its promise, DuP 697 was never commercialized because it turned out to be eliminated only very slowly from the body.
Soon after the prostaglandin conference the claimants had started work on developing diaryl heterocyclic anti-inflammatories using DuP 697 as lead compound. The object of their work was to discover another compound that was not only effective but also novel and hence patentable. The claimants found that:
- For cyclooxegenase activity the aromatic rings had to be located in adjacent positions on the heterocyclic ring.
- A CH3SO2 group in the p-position had to be present to inhibit the COX 2.
- The F atom on the other ring was not essential.
Their EP-B-679157 claimed a group of compounds said to exhibit anti-inflammatory or analgesic activity without causing erosion of the lining of the stomach because they selectively inhibit COX 2 (which produces inflammation) without substantial inhibition of COX 1 (which controls gastric secretion). The claimed compounds were therefore alleged to open up the possibility of an effective treatment for arthritis with reduced harmful side effects. They were of the formula:
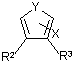
in which:
R1 and R2 represent aryl or heteroaryl which may be optionally substituted with a specified range of substituents, provided that one of them is substituted with methylsulfonyl (CH3SO2) or sulfamyl (NH2SO2);
X is hydrogen or is selected from a long list of possible substituents, including, amongst others, hydroxy (OH) and halogen (includes Br); and
Y is selected from S, O and –NR1- in which R1 is selected from hydrogen and alkyl.
The specification mentioned by name more than 140 compounds, of which 15 had been exemplified as being made (the remainder presumably being prophetic), all 15 had been tested in vitro for activity (IC50 against COX I and COX II) and five had been tested in vivo for anti-oedema or analgesia. No structure/activity relationship was disclosed except what could be gleaned from the claims. Unfortunately for the claimants, the most promising compound that they had identified within the class claimed in their patent was dropped because of poor bio-availability.
Merck had started work later than the claimants, but had succeeded in finding a compound MK 966 that was commercially exploitable, and that was of formula set out below:
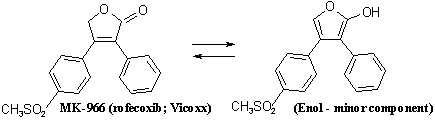
The claimants had not made MK-966 and had no specific claim in their patent directed to that product. They therefore had the more difficult task of establishing that one of the broad generic claims in their patent was valid and covered MK-966.
In defence to the infringement proceedings, Merck adduced experimental evidence that a number of the compounds covered by the patent did not have the claimed properties, including the materials of Examples 1, 2, 5, 11 and 15 of the patent in issue, three of which were held at the trial not to demonstrate any anti-inflammatory effect. One further compound that the defendants tested, referred to as Compound 68, had been listed in the patent as preferred. It had the formula:
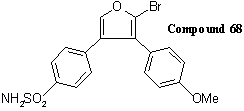
Despite being "preferred", Compound 68 proved to have all the undesirable features that the claimants aimed to overcome: it was selective for COX 1 and not COX 2, and it was a gastric irritant. The evidence included photographs of the gastric lesions that it caused. The claimants replied that the compounds made and tested by the defendants were not representative but had been carefully selected.
The Patents Court found that the patent was not valid and not infringed. The major conclusions of Pumfrey J. are summarized below:
- The invention with which the patent in issue was concerned was a class of compounds predicted to have anti-inflammatory activity and fewer or less drastic side effects owing to COX 2 specificity.
- A claim is invalid for insufficiency if covers subject matter that owes nothing to the invention disclosed. For a claim to a class of compounds to be valid, it must be possible to make a well-founded prediction that they have a common property that unites them as a class. "If compounds having the features of the claim may or may not possess the qualities which the patent says unify the class, it cannot be said that the claim represents a true class at all. It is just a generalized description of a large number of chemical compounds. Such a claim is not analogous to a claim to a new principle, since the patentee has given no information, such as a structure/activity relationship, which enables the reader of the specification to draw any conclusions as to the properties of any particular compound without further experiment. All he has done is to describe the scope of the claim with spurious precision."17
- The onus was on the claimants to show that the defendants’ test compounds were exceptional, and they had not done so, nor was there any substance in the defendants’ criticisms of their experimental technique. The experimental evidence showed that the properties of compounds within the claimed class were unpredictable, and that there were a substantial number of compounds that did not exhibit either COX 2 specificity or diminished side effects. The most distinctive feature of the claimed compounds was the 3,4-disubstitution rather than the 2,3-disubstitution of DuP 697, but this did not give success on its own, and it was not possible to identify features that did. Accordingly the patent was invalid for insufficient disclosure.
The above decision was affirmed on appeal to the Court of Appeal18 where Aldous L.J said:
"The patent in this case claims a class of compounds. There is no technical contribution in a list of compounds which a skilled person would know how to make at the priority date. The 20 year monopoly was granted because of the disclosure in the specification that the class of compounds claimed had the quality disclosed in the specification. The invention or technical contribution justifying the monopoly claimed can only be that quality. I have already decided that the judge was right when he held that the specification would be read by the skilled person as disclosing that the claimed class of compounds had anti-inflammatory and/or analgesic effect with fewer and less drastic side-effects, the reduction in side-effects being due to Cox II selectivity. It is that disclosure which is the technical contribution and invention ...
I agree with the judge. He could have gone further and pointed out that the claims covered an enormous number of compounds and that upon his findings many hundreds if not thousands did not have the quality of the class. For example, the list of possible substitutions for X is extremely large and a very large number would be inactive because they are too large. As pointed out by Professor Baker, many of that group can be bigger than the rest of the molecule. The term "aryl" is unbounded and the term "aryl" introduces groups which are at least as large as the rest of the molecule itself."
In parallel proceedings before the EPO, EP-B-0679157 was revoked on the ground that all the requests (sets of amended claims) submitted by the patentees related to intermediate generalizations that were not supported by the application as filed and hence contravened article 123(2) EPC19.
American Home Products and Professor Sir Roy Calne v Novartis20 – You may be in trouble if you don’t Markush!
Rapamycin is a macrolide of the formula shown below. It was known prior to Professor Calne’s invention as an antifungal antibiotic of formula:
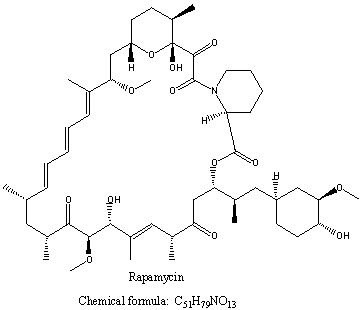
Professor Calne’s contribution was to discover that rapamycin could also be used as an immunosuppressant. The patent in issue was EP-B-040174721, and its main claim read:
"Use of rapamycin for the preparation of a medicament for inhibiting organ or tissue transplant rejection in a mammal in need thereof."
There was, however, a broadening statement in the description, which read:
"The present invention includes the use of natural and synthetic rapamycin, genetically engineered rapamycin, and all derivatives and prodrugs of rapamycin, such as described in ... US Patent Nos. 3929992, 3993749, 4316885 and 4650803."
The defendants’ compound had been made by replacing the OH group on the ring of the side chain with a 2-hydroxyethyl group. Trial of the present infringement proceedings had (presumably by agreement) covered only issues concerning infringement and sufficiency of disclosure, leaving other issues such as novelty and inventive step to be considered later if so required. The questions before the court were whether:
- The claim covered the defendants’ compound, which was not rapamycin but instead was 40-O-(2-hydroxyethyl)-rapamycin.
- The patent was bad for insufficiency if the claim did so. Possible grounds of insufficiency were that the claim covered a class of compounds whose scope was impossible to determine, and that in order to discover rapamycin derivatives within the scope of the claim a skilled person would have to embark on a prolonged and unduly burdensome research program.
- There was a distinction between ordinary claims and "second medical use" claims.
Laddie J handed down an opinion of the Patents Court on 6th December 1999. He held that the claims covered the defendants’ derivative and rejected the arguments concerning insufficiency, i.e. because of the fundamental nature of Professor Calne’s discovery it was legitimate for his patent to "reach-through" to other structurally similar compounds discovered by subsequent empirical research. His opinion made the following points:
- The word "rapamycin" used in the claims meant a single known chemical and did not mean "rapamycin and derivatives of rapamycin which exhibit rapamycin-like immunosuppressant activity."
- There was a distinction between the meaning of the words used in a claim and the meaning of the patent. In the present case, as in Kastner v Rizla22, the inventor had not made it clear that his intention was only to seek limited protection, but instead there were factors e.g. the statement about derivatives and prodrugs that showed that the intention was to cover more than rapamycin. If the specification were limited to a single compound, it would be virtually valueless because: "It would have disclosed to the art the novel seam of interrelated molecules but have claimed only one of them." Any reasonable addressee would have been surprised at such a limitation. Instead, the patent covered those derivatives of rapamycin that produced the same type of inhibition of organ rejection as did rapamycin itself.
- Because there was no sure way of predicting which derivatives would be effective, the inventor should not be forced into a choice between accepting narrow protection and putting forward a wider claim with neatly defined boundaries but where the appearance of certainty was an illusion and the claim was in truth a calculated gamble. "Where the technology makes prediction impossible it cannot be right that the law requires it."
- (after reviewing Biogen v Medeva23 and Genentech I/Polypeptide expression24). There is nothing wrong in principle in the patent covering both rapamycin and derivatives that work in the same way. "Sufficiency requires the monopoly to match the contribution. The fact that here, as in most if not all other pharmaceutical cases, there is no way of predicting with accuracy which derivative molecules will possess new and improved properties does not mean that a monopoly which covers all rapamycin derivatives which work extends beyond Professor Calne’s contribution. On the contrary, although his discovery of the effectiveness of rapamycin was, no doubt, empirical, once that discovery had been made, it made available to the art the opening through which a new class of immunosuppressants could be found. The discovery of other molecules which achieve the same or better results is no longer empirical. The addressee of the patent can work in a logical and predictable way from the one molecule disclosed in the patent to the similar molecules, which are derived from it. Unlike the examples given in Biogen, this patent is not attempting to cover all new immunosuppressants. It is directed only to those molecules which can be reached as a result of rapamycin having shown the way."
- The research necessary to find further derivatives did not impose an undue burden because the required iterative work was standard and expected for finding good candidates in the pharmaceutical industry, and the time and money required was only an indication that the research in this field was slow and expensive. However many derivatives had been disclosed in the patent, it could still be argued that the same process of synthesis and testing would be required to find further derivatives. If the only safe course were to limit the patent to the molecules that had actually been tried and tested, that would make patents for pharmaceuticals more or less valueless.
- The second use form of claim should make no difference to the outcome. "If Professor Calne had happened to find his novel use for rapamycin before it had been found to have fungicidal properties, his claims would not have needed to be in second use form. However, the invention would have been exactly the same and the requirement for sufficiency would have been exactly the same."
The Court of Appeal agreed with the High Court that "rapamycin" when used in the claims referred to the single known molecule and did not also refer to derivatives that also inhibited organ rejection. Aldous L.J. based his conclusion on a linguistic analysis of the specification, which showed a distinction was made between rapamycin and its derivatives, and on the fact that the specification did not disclose a single derivative that had been shown to work.
He went on to find on the basis of the "Protocol questions"26 that the defendant’s compound was not a variant falling within claim 1 of the patent in issue. The defendant’s compound did not satisfy the test imposed by the second Protocol question, which in the interests of third parties required for an affirmative answer that the variant should be obviously or clearly immaterial. In the present case, although the defendant’s compound might have been a good candidate to try, it was not obvious that it would work as an immunosuppressant and in order to find that out you would have had to make and test the compound, which required research. The inventor had only discovered and described the second medical use of rapamycin, and it was left to others to find out which derivatives, if any, worked. Furthermore, it was unfair to the patentee to construe his claim in a way that was not intended. To ignore a limitation could render a patent invalid contrary to the wishes of the patentee. In answer to the third Protocol question, the skilled person would have understood that strict compliance with the primary meaning was essential because:
- throughout the specification "rapamycin" was used to denote the molecule itself, and it would be surprising if a different nomenclature was used in the claims;
- the inventor’s discovery had been defined in relation to rapamycin itself;
- if the patentee had intended to cover derivatives, he could easily have done so; and
- a claim covering rapamycin and rapamycin-like derivatives would not have been allowed by the EPO as it would have lacked support and been speculative. The broadening passage relied on by the claimants contained an unqualified reference to all derivatives and prodrugs of rapamycin whereas the EPO would not have permitted all derivatives to be claimed27. Furthermore a test or standard was needed by which a skilled person could decide whether or not a particular derivative had a rapamycin-like effect and the specification did not disclose any such test.
If claim 1 had the broader scope for which the patentees had argued, then Aldous L.J. concluded that the specification would be insufficient. The requirements for sufficiency were (a) that the claim should be enabled over its whole scope28 and (b) the skilled person should not be required to carry out research to ascertain how the invention is to be performed29 (though he could be required to use appropriate skill and tenacity). In this case, if the patent had covered derivatives of rapamycin, it would have needed to teach how to perform the invention with such derivatives. The specification did not do so because although it told the skilled man where to start, it left him to ascertain by research which derivatives work, the number of derivatives was vast and the task of ascertaining those which would satisfy the functional part of the claim was vast and correspondingly burdensome. A claim covering derivatives would cover all molecules that would work while leaving it uncertain which ones did and how many of them there were. Such a claim did not reflect a class with a unifying characteristic but was a claim to a number of compounds with the number and identity being left to the skilled person to find out.
It was not true that a patent covering only a second medical use for rapamycin would be virtually valueless. The patent protected that use and the long and expensive work needed to obtain regulatory approval. Anyone who wished to market a derivative would have to make the derivative and carry out the long and expensive work to get the derivative on the market. The patent system should not be used to enable a person to cover more than he had described in sufficient detail to amount to an enabling disclosure, and in this case it would stifle research aimed at finding a derivative of rapamycin that was a better immunosuppressant than rapamycin itself. In support of the latter proposition Aldous L.J. quoted as apt a passage from the Biogen case in which Lord Hoffmann discusses the work of Samuel Morse and the Wright Brothers and concludes that:
"...It is inevitable in a young science, like electricity in the early nineteenth century or flying at the turn of the last century or recombinant DNA technology in the 1970s that dramatically new things will be done for the first time. The technical contribution in such cases deserved to be recognised. But care is needed not to stifle further research and healthy competition by allowing the first person who has found a way of achieving an obviously desirable goal to monopolise every other way of doing so..."
The defendants’ compound contained up to 0.8% of rapamycin and the patentees argued that this was sufficient to establish infringement. Aldous L.J. rejected this argument on the ground that the medicament had to be essentially rapamycin and there had been no discovery that medicaments containing only 0.8% rapamycin had any therapeutic effect. Accordingly the Court of Appeal found that the patent was valid but not infringed.
Conclusion
In an empirical research field, and in particular in the pharmaceutical field, it is difficult for a pioneering inventor to formulate a generic claim that is both wide and scientifically supportable because there are no established structure/activity relationships that can be used as a basis for rational prediction. The difficulties are illustrated by the Rapamycin case where Professor Calne had researched the prior art concerning rapamycin derivatives, but that prior art was relatively sparse and potential sites for modification and the range of permissible changes at those sites were only beginning to be investigated. It was only after Professor Calne’s invention that significant numbers of patents disclosing structure/activity investigations for compounds within the rapamycin family started to appear. Similarly in the Monsanto case, although pharmaceutically active disubstituted thiophenes were known, the COX-2 target was relatively new and there was no body of knowledge concerning previous selective inhibitors of COX-2 on the basis of which valid predictions could be made. A reader of the specification would be struck by the limited information as to activity supported by reports of actual tests, and might view the claim scope as driven more by insight as to what was chemically available than insight as to biological effectiveness. This situation may be contrasted with that in Olin Mathieson v Biorex where a specific change was being made at a particular position within a well-explored family of compounds, and an existing body of knowledge of structure/activity relationships in parts of the molecule that were not subject to change provided basis for a claim of useful generic scope that nevertheless survived the challenge of litigation.
Two drafting points emerge. Firstly, as shown by the Agrevo and Monsanto decisions discussed above, when dealing with inventions concerning biologically active compounds, including open-ended substituent definitions e.g. alkyl or aryl in a generic claim is risky and should be backed-up by other claims with more specific generalisations in which the maximum number of carbon atoms is specified or e.g. an overall range of molecular weights is specified. Secondly, if the invention involves a specific change at a location in a known family of molecules, then it may be to the applicant’s advantage to discuss in detail the results achieved in structure/activity relationships at other locations within the molecules described in the prior art because this helps to show that in these aspects the genus claimed is not an irrational prediction but builds on previous successful experimental work, as in Olin Mathieson above.
There is no fully satisfactory way of claiming generic pharmaceutical inventions. The Markush approach has at least 80 years of use, but the validity of Markush claims if challenged by a resolute and well-resourced opponent or defendant should not be taken for granted. No judge is likely to treat a generic claim sympathetically following demonstrated inactivity within the genus. Over-broad claims should be avoided, and participation not just from chemists but also from biologists and molecular modellers can be helpful. Furthermore, it is important to claim the disclosed compounds individually as well as within the genus because the claims to the individual compounds may be the only valid claims.
Footnotes
1.Attributed to Ernest Rutherford. Notwithstanding his preference for systematic over descriptive knowledge, the lives of most readers will have been saved at least once by the descriptive knowledge embodied in a pharmaceutical product.
2. Attributed to Thomas A. Edison.
3. Richard P Feynman, "Cargo Cult Science", Surely You’re Joking Mr. Feynman, Vintage, 1992 (adapted from the Caltech commencement address given in 1994). This short paper is a classic which merits periodic re-reading and reflection.
4. (1928) 45 RPC 153
5. 383 U.S. 519 (1966)
6. Consolidated Electric Light Co v. McKeesport Light Co, 159 U.S. 465 (1895)
7. 67 RPC 23 at 50
8. Lord Normand at p. 38
9. [1997] RPC 1 at page 49 lines 1 to 9.
10. The existence of an unexpected new effect may be a necessary condition for patentability but it is not a sufficient condition. If the prior art when considered collectively suggests some other advantageous new effect, then arguments concerning mere "bonus effect" or "one-way street" become relevant, and inventive step may be denied.
11. G.S.A. Szabo, "The Problem and Solution Approach to the Inventive Step", [1986] 10 EIPR 293-303.
12. T 0939/92,
13. [1970] RPC 157
14. By modern standards the supporting disclosure of the patent is sparse. The introduction does not discuss the prior art, and the presentation of the invention is almost exclusively chemistry-oriented with only a brief value statement. Although comparative activity of the claimed compounds relative to chlorpromazine is discussed, no procedure is disclosed for measuring activity, and animal models for the indicated diseases are not mentioned. Dosages are related to that for chlorpromazine adjusted for relative activity. Pharmaceutical compositions are neither disclosed not claimed, which is a surprising omission given the advantages of such field-specific or medical indication-specific claims if the patent becomes the subject of litigation.
15. [1996] FSR 153
16.
http://www.courtservice.gov.uk/judgmentsfiles/j455/patents_monsanto.htm, [2000] RPC 77.17. The following earlier UK decisions were referred to on the issue of sufficiency: British United Shoe v Simon Collier (1910) 27 RPC 567, May & Baker v Boots (supra), Olin Mathieson v Biorex (supra) and Biogen v Medeva (supra).
18.
http://www.courtservice.gov.uk/judgmentsfiles/j413/Pharmacia_v_Merck.htm19. See T 0812/00 Thiophenes/Searle et al dated 26 February 2002.
20. [2000] RPC 547
21. Some indication of the significance of its contribution can be gauged from the fact that his equivalent US Patent 5100899 at the time of writing has 121 forward references according to the Delphion database. Rapamycin has been found to have a novel mechanism of immunosuppression to prevent rejection of organ transplants. Treatments conventionally used, such as cyclosporin and FK506 are effective in ensuring the short-term survival of the transplant, but the organ is rejected in the long term. It gives rise to a reduced incidence of acute rejection episodes, lower GFR at one year post-treatment and appears to cause fewer side effects than the standard anti-rejection treatments.. It is now marketed under the tradename 'Rapamune' by Wyeth. A visit to the Novartis website has not shown that the rival 2-hydroxyethyl compound has yet matured into a product.
22. [1995] RPC 585
23. [1997] RPC 1
24. T 0292/85; [1989] OJ EPO 275
25. Valensi v British Radio Corporation [1973] RPC 7, Mentor v Hollister [1993] RPC 7, T 0032/85 Gist-Brocades/Biomass [1986] 5 EPOR 267 and T 0226/85 Unilever/Stable bleaches [1989] 1 EPOR 18 considered. Note that during the appeal stage during the Viagra litigation in the UK, Pfizer successfully submitted in relation to a generic claim to inhibitors of the PDE 5 enzyme: "Once you know what you are looking for, you can get it and screening does not involve inventive effort or undue labour.", see Lilly Icos v Pfizer [2002] EWCA Civ 1,
http://www.courtservice.gov.uk/judgmentsfiles/j507/Lilly_Icos_v_Pfizer.htm26. These were formerly called the Catnic questions and as currently formulated are: (1) Does the variant have a material effect upon the way that the invention worked? (2) Would it have been obvious to a skilled person that the variant would not have a material effect on the way the invention worked? (3) Would the skilled person have understood from the language of the claim that the patentee intended that strict compliance with the primary meaning was an essential requirement of the invention?
27. Aldous L.J. does not give a reference in support of the proposition that it is objectionable in claims to chemical compounds to cover all possible substituents, but presumably he had in mind the well-known EPO Appeal Board decision in T 00939/92 AGREVO/triazoles
28. Biogen Inc v Medeva Plc [1977] RPC 1
29. Mentor Corporation v Hollister [1993] RPC 7
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.

