Gene therapy is considered to be one of the most exciting and promising forms of medical treatments available today, due to continuous breakthroughs in research and increasingly positive clinical trial results. In this article, we take a look at some of the latest successes in gene therapy medicines, as well as recent patent filing trends in the gene therapy sector.
What is gene therapy?
Gene therapy is a medical technique which uses genetic material to prevent, treat and potentially even cure disease, by correcting the underlying genetic problem in the patient. The approach enables the treatment of a disease by altering the patient's own genetic make-up, rather than using surgery or administering pharmaceuticals. The genetic material, which may be either DNA or RNA, is introduced into the patient's faulty target cells using a vector, for example an adeno-associated virus (AAV) vector, and so encodes the genetic instructions to repair how a defective or depleted protein (or group of proteins) is produced by the cells in order to render them healthy.
Gene therapy can generally be delivered in two ways. In one scenario, the genetic material is administered directly into the patient (in vivo). Another approach, however, is to remove a person's own diseased cells, into which the new and corrected genetic material is delivered outside of the patient's body (ex vivo) and these modified cells are then returned back into the body. The result of either approach is that the genetic make-up of the patient is altered to result in a corrected protein(s), and, in so doing, treat or cure the disease.
Recent gene therapy success stories
It is reassuring to see that there have been a number of successes recently in the field of gene therapy medicines, notably with the following three examples:
In 2024, Eli-Lilly's hearing loss gene therapy drug, AK-OTOF, successfully enabled an 11-year-old boy who was born with sensorineural hearing loss (SNHL) due to a mutation in the otoferlin gene, to hear within a normal range. The patient received a single, unilateral intracochlear dose of AK-OTOF, which is a dual adeno-associated (AAV) vector-based gene therapy.
AK-OTOF is designed to restore auditory function by gene transfer and durable expression of normal, functional otoferlin protein to the inner hair cells of the cochlea. Eli Lilly have reported that, within only 30 days, the patient's hearing was restored across all tested frequencies, achieving thresholds of 65 to 30 dB HL.
The patent family aiming to protect AK-OTOF, has a series of patents and patent applications in multiple jurisdictions, based on the PCT application WO2018/039375, entitled "Compositions and Methods for treating non-age-associated hearing impairment in a human subject", filed in 2017. The European application (EP3510161A) belonging to this family is currently pending, with claims drawn to the second medical use of a composition comprising at least two different AAV vectors for treating hearing loss. The first AAV vector comprises a coding sequence that encodes an N-terminal portion of an otoferlin protein, and the second AAV vector comprises a coding sequence that encodes a C-terminal portion of an otoferlin protein. This patent family also includes two granted US patents (US 11,781,145 B2 and US 11,525,139 B2), protecting the dual vector constructs per se, and a method of treating otoferlin related hearing loss with the dual vector constructs.
Sickle cell disease & Beta thalassemia
In 2023, the Medicines and Healthcare products Regulatory Agency (MHRA), the European Medicines Agency (EMA), and the US Food and Drug Administration (FDA), all recommended approval for the drug, CasgevyTM, for the treatment of two blood disorders, sickle cell disease (SCD) and beta thalassemia.
Sickle cell disease and beta thalassaemia are two inherited rare genetic conditions caused by genetic mutations in the genes encoding haemoglobin, which is an essential protein found in red blood cells, and is responsible for carrying oxygen around the body. The therapy, developed through a partnership between Vertex Pharmaceuticals and CRISPR Therapeutics, is based on a non-viral, ex vivo CRISPR/Cas9 gene therapy. This is the first treatment to be licensed using the CRISPR gene-editing tool, for which its discoveries were awarded the Nobel prize in 2020.
The therapy first involves removing bone marrow stem cells from the blood of a patient. The CRISPR gene editing tool then introduces a precise double-strand break into the DNA at the erythroid-specific enhancer region of the BCL11A gene, which usually prevents the production of foetal haemoglobin (HbF). These CRISPR-modified cells are then infused back into the patients, and the reduction of BCL11A gene transcription caused by the gene editing leads to an increase of HbF production, which allows the patient to now produce functioning haemoglobin, and so treats sickle cell disease (SCD) and beta thalassemia.
There appears to be several patent families with applications covering the CasgevyTM therapy, based on PCT applications WO2017/077394, WO2017/182881 and WO2019/113149. In Europe, EP3371306 B1 (based on WO2017/077394) has granted, with claims drawn to a method of genome editing using a Cas9 DNA endonuclease to effect a double-strand break at one or more loci within the β-globin region of human chromosome 11, using guide RNAs with specific spacer sequences. EP3445388A (derived from WO2017/182881) is intended to be granted soon, with claims drawn to a single-molecule guide RNA (sgRNA) comprising a specific nucleic acid sequence and three 2'-O-methyl-phosphorothioate residues at each of its 5' and 3' ends, as well as its use in treating a patient with hemoglobinopathy (e.g. sickle cell anaemia or thalassemia). Finally, EP3720473A (based on WO2019/113149) is still pending, with the claims currently relating to a specific dose of a composition comprising CD34+ human hematopoietic stem and progenitor cells (hHSPCs), comprising a genetic modification within a +58 DNase I hypersensitive site within the erythroid lineage-specific enhancer of a BCL11A gene.
Hereditary angioedema (HAE), which is thought to affect about one in 50,000 people, is a genetic disorder characterised by severe, painful and unpredictable swelling attacks, caused by a genetic mutation that leaves patients with leaky blood vessels. The disorder can affect the airways, and therefore, an individuals' ability to breathe, and can, in some cases, be fatal. HAE is caused by the overactivity of the enzyme kallikrein, which drives multiple inflammatory pathways, including the production of the inflammatory mediator bradykinin, which is overproduced in HAE.
Researchers from the University of Auckland, Amsterdam University Medical Center and Cambridge University Hospitals have successfully treated more than ten patients with the CRISPR/Cas9 gene editing therapy, called NTLA-2002. This approach utilises an in vivo CRISPR/Cas9 technology, in which the CRISPR editing machinery is packaged into nanoparticles that are designed to target the liver, where the excess kallikrein proteins are produced. The CRISPR editing machinery then targets the KLKB1 gene, which is responsible for the overproduction of plasma prekallikrein, and disables it. By editing this gene, the therapy reduces the levels of total plasma kallikrein and halts the production of bradykinin, effectively preventing angioedema (swelling) attacks.
The patent family relating to NTLA-2002 is based on PCT application WO2021/158858, entitled "Compositions and methods for kallikrein (klkb1) gene editing", filed in 2021. The claims of the pending European application belonging to this patent family are drawn to a guide RNA comprising a guide sequence designed to target the KLKB1 gene, as well as a composition comprising the guide RNA and Cas9, and its use in treating HAE.
Gene therapy patent filing trends
A patent search conducted on Espacenet (the European Patent Office's patent search tool) in the field of gene therapy (IPC Classification A61K 48/00, "Medicinal preparations containing genetic material which is inserted into cells of the living body to treat genetic diseases; Gene therapy"), revealed that there has been a steady increase in the number of gene therapy patent applications in the past 10 years, as shown in the graph below.
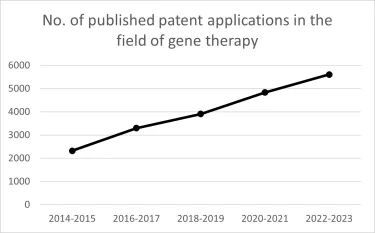
For example, between 2014 and 2015, there were 2320 publications of patent applications in the field of gene therapy. However, this number has steadily increased year on year, with a total of 5605 publications occurring between 2022 and 2023. With the significant growth and breakthroughs of gene therapy innovations in recent years, it will be exciting to see how the number of corresponding patent filings develops over the next few years.
Venner Shipley has a team of patent attorneys who specialise in drafting and prosecuting patent applications in the gene therapy space. The team is experienced in handling patent applications in technology areas, including those related to gene delivery vectors (e.g. AAV vectors), gene and protein engineering, gene editing (e.g. CRISPR), modified genes and expression products, and control of gene expression to name a few. Our team handles oppositions, and is also experienced in providing freedom-to-operate advice, in the field of gene therapy.
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.

