When it comes to protecting technical innovations there are two broad strategies: keep it a trade secret or obtain a patent. It has long been the case that computer-implemented technologies and pharmaceutical technologies are technical fields where one or the other approach (trade secrets in the case of computers, patents in the case of pharmaceuticals) has been typically favoured. So, in the brave new field of applying advanced computational systems to the development of pharmaceuticals, how do we develop a strategy which satisfies both approaches to protecting technical innovations?
To do so, we must look at the reasoning behind the preference for each strategy and some of the problems faced by proprietors when protecting their innovations in these technical fields.
There can be little doubt that sophisticated dynamic computational systems, such as those dubbed "Artificial Intelligence" (AI) and "Machine Learning", are being developed for use in all stages of drug design and development. Advanced computational systems are being used to reduce the costs associated with drug development, increase the number and variety of candidates for further testing and improve testing and screening of existing candidates, to name a few example applications.
Recent reports in the press would indicate that AI systems are getting reasonably powerful at developing new and potent biologically active compounds. For example, according to a report by The Verge "AI suggested 40,000 new possible chemical weapons in just six hours".1 In this case, researchers were exploring how artificial intelligence could be used to develop biochemical weapons. Their findings were published in the journal "Nature Machine Intelligence".2
So, what sort of strategy should be adopted for a system that can potentially identify 40,000 biologically active compounds in six hours and what about the biologically active compounds themselves?
Patents
The rise of the application of AI and machine learning in the fields of healthcare and drug development has inevitably led to a corresponding rise in patent applications in this crossover field. Therefore, questions regarding what and how to effectively protect developments made in improving and adapting AI and machine learning in various aspects of pharmaceutical development are being frequently raised and discussed.
Where these computational systems assist inventors in a meaningful way, it follows that there must be some technical and patentable consideration of what parameters of the systems are required for the effective use of the system. The development and suitability of such systems for drug development must, many argue, require technical and practical consideration.
Not only do the computational systems themselves represent considerable value to applicants and inventors but also the ability of such algorithms, software, or systems to develop new candidates for drug therapies, screen existing candidates, provide in silico models, or develop diagnostics (to name a few). In other words, there is real value in the output of the system as well as the system itself. However, claiming the output of the system based on the virtue of the system itself runs headlong into well-established principles of patent law.
Issues Relating to Patents
Patents and patent applications regarding AI and machine learning face their own challenges. In 2018, the European Patent Office (EPO) amended their Guidelines for Examination to include a section on artificial intelligence and machine learning making clear that such inventions would be considered as other computer-implemented inventions and assessed under the EPO's well-established framework considering technical character, technical contribution and technical effect of the subject matter of the invention.
G-II, 3.3.1 of the 2022 edition of the EPO's Guidelines for Examination provides the following guidance on potential technical application for AI and machine learning:
"Artificial intelligence and machine learning find applications in various fields of technology. For example, the use of a neural network in a heart monitoring apparatus for the purpose of identifying irregular heartbeats makes a technical contribution. The classification of digital images, videos, audio or speech signals based on low- level features (e.g. edges or pixel attributes for images) are further typical technical applications of classification algorithms."
Meanwhile, in 2020, the United States Patent and Trademark Office (USPTO) released a report on "Public Views on Artificial Intelligence and Intellectual Property Policy", which also indicated that "AI inventions should not be treated any differently than other computer-implemented invention". Thus, the USPTO determines patent-eligibility of AI and machine learning related inventions based on the existing test which evaluates whether such inventions fall into the judicial exceptions, such as being an abstract idea. Finding that an invention has a practical application often helps to ensure that the invention falls outside the judicial exceptions, and thus is patent-eligible.
The patent system therefore appears to be appropriate for protection of innovation relating to AI and/or machine learning systems which are applied to the practical and technical problems associated with pharmaceutical development.
It is worth noting that the utilisation of AI and machine learning systems in pharmaceutical development, as with other computer-implemented inventions, may have a stronger and more straight forward case for falling outside the judicial exceptions in the US than being found to provide a technical solution to a technical problem as required at the EPO. Some systems may therefore have a better chance before the USPTO than the EPO and as such may require different patentability considerations for each jurisdiction.
Claims to Products Resulting from an AI/ ML Process
Patents appear to be a suitable means for protecting the underlying AI or machine learning system directed to solving a practical or technical problem in developing or screening pharmaceutical products, but what about the products themselves?
Both the USPTO and EPO require some structure or technical content to be imparted on the product by the inventive process in order for such product claims to be found patentable.
Guidelines for Examination at the EPO recite:
"The technical content of the invention lies not in the process per se, but rather in the technical properties imparted to the product by the process." (F-IV, 4.12)
Whilst the Manual of Patent Examining Procedure at the USPTO provides:
"Product-by-process claims are not limited to the manipulations of the recited steps, only the structure implied by the steps." (Section 2113)
Therefore, claims directed to the products of the AI or machine learning systems must have some structural and technical property imparted on them by virtue of being produced by the AI or machine learning system which renders the product novel and inventive in its own right.
Application of Known AI/ML Process to Novel Purpose
A further consideration for the application of AI or machine learning processes in the development of pharmaceuticals is the application of such processes in which the process is known but the application is novel.
At the EPO, the novel purpose of a known process would have to apply such a known process in a non-obvious way and have for example a new and surprising effect or would have to overcome technical difficulties not resolvable by routine techniques.
AI/ML as a Routine Technique
AI or machine learning inventions directed to developing new drugs or otherwise deriving their technical character from undisclosed applications and effects run a high risk of facing patentability issues in the EPO or AI or machine learning inventions directed to developing new drugs or otherwise deriving their technical character from undisclosed applications and effects run a high risk of facing challenges in the EPO. The EPO would consider whether the disclosure is sufficient that it was plausible that such effects would arise or that such effects arise across the entire scope of the claims.
The challenge for inventions of this nature will be to navigate inventive step and sufficiency requirements. The applicant must try to show that the skilled person would understand the undisclosed and technical application well enough to implement the invention. The applicant must also prove that the functional elements of the system are sufficiently distinct from the prior art meaning the skilled person would not find the system itself to be obvious over the prior art.
In other words, could generic or known AI systems be considered tools for routine experimentation like other known tools and thereby render a novel and inventive application of such tools plausible?
If so, are there aspects of such systems that are not routine and have a new and surprising effect? An applicant must consider what elements of the utilised AI/machine learning system are known or routine and what elements differ in their application sufficiently to be suitable grounds of a patent application. Above all else, such a distinction must be described in sufficient technical detail and the new and inventive patentable aspects delineated from that which is known or routine.
Trade Secrets and Data Access
There are valuable aspects of the innovation associated with computerised systems implemented in the development of valuable end products that may not be suitable for the patent system alone in its current form.
Provisions for trade secrets provide an alternative and/or supplementary mechanism to the patent system for protecting innovations of a technical nature.
The protection afforded by trade secret provisions is automatic for as long as the requirements of the relevant trade secret laws are met e.g., keeping the relevant information secret.
However, technology that requires regulatory approval (and therefore detailed disclosure), may not be suitable for complete reliance on trade secret provisions. Pharmaceutical technology, for example, cannot rely on trade secret provisions to the extent that the technology needs to be disclosed to regulators for market approval.
US and European Trade Secret Law
In the US, a trade secret is information that the proprietor has taken reasonable steps to keep secret and that has commercial value by virtue of it being a secret. However, as mentioned above, protection only lasts for as long as these conditions are met.
Information would stop being a trade secret if it ceased to be a secret or if the owner failed to maintain reasonable measures to maintain its secrecy.3
Similar standards for trade secrets have been introduced into European law by EU Directive (2016/943).
In accordance with the EU Directive 2016/943 on trade secrets, all EU members at the time (including the UK) unified their provisions for the protection of trade secrets and brought their requirements largely in line with those in the US.
Namely, the requirements that a trade secret be information that is (i) secret (ii) has commercial value because it is secret and (iii) has been subject to reasonable steps under the circumstances to keep it secret (see Article 2 of EU Directive 2016/943).
A key consideration, therefore, for highly valuable information relating to technology for the development of pharmaceutical products is whether the relevant information can be successfully kept secret and what reasonable steps must be maintained.
Pharmaceutical innovations usually have an associated obligation of disclosure to regulators and remain commercially significant throughout the term of a patent and beyond, whilst computer-implemented technologies can be made difficult to reverse engineer (such as with SaaS models) and access can more easily be restricted so the risk of inadvertent disclosure to the public can be significantly reduced. Computer- implemented technologies are also more often made redundant before the end of the term of a patent.
Figure 1 below illustrates an example IP strategy for an AI or Machine Learning technology with a pharmaceutical application:
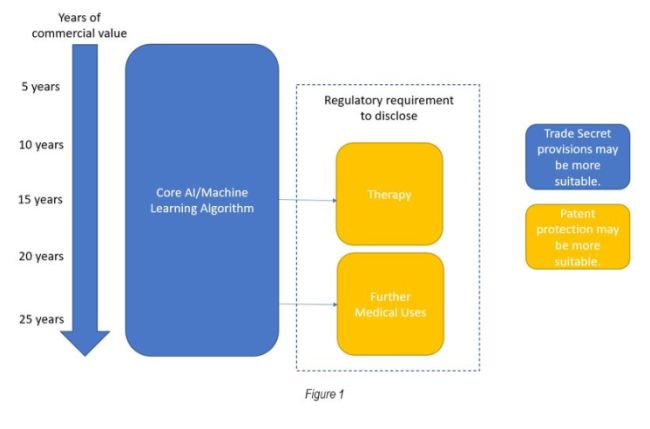
In relation to taking reasonable steps for technology that is suitable for being kept secret, examples of reasonable steps may include: identifying and labelling secret information; informing and agreeing confidentiality with those who have access; and managing security risks such as encrypting the relevant information.
As with all matters of legal enforcement, the collection and maintenance of evidence is key. In the case of trade secrets this means data about the data or metadata should be collected and maintained.
It should also be noted that, according to a research article from the University of Oxford, the more widely information is shared within an organisation, the less time until eventual disclosure.1 Table 4 of the article indicates that to keep something secret for 20 years, no more than 628 people can have knowledge of the secret.
Strategic Considerations
In consideration of all of the above, a strategy including patent and trade secret provisions across both Europe and the US is most likely to be the best way to sufficiently protect valuable developments of AI and machine learning systems in the field of pharmaceuticals.
A significant consideration in such a strategy should balance the practicalities of protecting secret information from unauthorised disclosure over time and the suitability of information for patent protection.
Where possible and when trade secret laws are being relied upon, access to information should be limited on a need-to- know basis and where there is a significant risk of disclosure or leaking of information a patent strategy should be formulated.
Footnotes
1. https://www.theverge.com/2022/3/17/ 22983197/ai-new-possible-chemical-weapons-generative-models-vx
3. For more on legal protections for US trade secrets please see here: https://www.finnegan.com/a/web/316168/PUBLISHED-Bloomberg- Law-Legal-Protections-for-U.S.pdf
4. https://journals.plos.org/plosone/article?id= 10.1371/journal.pone.0147905 [On the Viability of Conspiratorial Beliefs – David Robert Grimes, University of Oxford]
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.


