A recent U.S. District Court ruling will likely have far-reaching implications for determining Patent Term Adjustment (PTA). On November 1, 2012, the U.S. District Court for the Eastern District of Virginia issued a decision in the case of Exelixis, Inc. v. Kappos (Case No. 1:12-cv-00096), ruling that a Request for Continued Examination (RCE) filed in a patent application more than three years after the effective filing date of the application has no impact on PTA as determined under 35 U.S.C. § 154(b) ("the PTA statute"). Usually, an RCE reduces the PTA, but the district court ruled that if initiated more than three years after the effective filing date, an RCE would not reduce the PTA at all. This will potentially extend the term of some recently issued patents and numerous to-beissued patents. Some commentators have estimated that this ruling could impact approximately 10% of recently issued patents. Depending on the patent, the effects of the decision could amount to additional PTA of days, weeks, or in some cases, even years.
Requests for Continued Examination (RCEs)
RCEs are a commonly-used procedural mechanism in patent prosecution that was added as part of the American Inventors Protection Act of 1999 (AIPA). RCEs allow applicants to continue examination of an application before the Examiner rather than appealing to the Patent Trial and Appeal Board. Put simply, RCEs provide applicants with a proverbial "second bite at the apple," offering the opportunity to file, for example, additional substantive amendments and arguments after receiving a final rejection on the merits from the Examiner. RCEs are attractive because they allow continued negotiation with the Examiner and tend to be cheaper and faster than the appeals process.
Patent Term Adjustment
The AIPA significantly changed how delays during prosecution of a patent application affect the 20-year term of any issued patent, which is measured from the earliest U.S. filing date. The PTA statute provides "guarantees" against various types of U.S. Patent and Trademark Office (USPTO) delays and also reduces any accumulated PTA based on delays attributable to the Applicant. Generally speaking, USPTO delays under the PTA statute fall into three categories:
"A" delays (Section 154(b)(1)(A)) – which accrue when the USPTO does not act within certain statutory timeframes (e.g., issuing an Office Action within 14 months after the filing date);
"B" delays (Section 154(b)(1)(B)) – which accrue when the USPTO fails to issue a patent within three years after the filing date of the application in the United States; and
"C" delays (Section 154(b)(1)(C)) – which accrue when an application is delayed due to an interference, a secrecy order, or an appeal.
The total PTA is the sum of any "A," "B" and/or "C" delays excluding (i) any overlap between these delays and (ii) any delays attributable to the Applicant. Wyeth v. Kappos, 591 F.3d 1364 (Fed. Cir. 2010).
In particular, Section 154(b)(1)(B), provides a guarantee of no more than a three-year application pendency, subject to certain exceptions. Subparagraph (B)(i), for example, excludes from the three-year guarantee "any time consumed by continued examination," that is, via the filing of an RCE.
Since the passage of the AIPA, the USPTO has interpreted Subparagraph (B)(i) as requiring that time consumed by an RCE is always excluded from the calculation of "B" delay, regardless of when the RCE is filed. See 37 C.F.R. §§ 1.702(b)(1), 1.703(b)(1).
Exelixis' Challenge to the USPTO's Interpretation of Section 154(b)(1)(B)(i)
Exelixis, Inc. ("Exelixis") is the owner of U.S. Patent No. 7,989,622 ("the '622 patent"), entitled "Phosphatidylinositol 3- Kinase Inhibitors and Methods of Their Use." In short, the '622 patent is directed to small molecule inhibitors of protein kinases (a type of enzyme) associated with certain types of cancer and to methods for treating, preventing, and/or inhibiting these types of cancer. Exelixis challenged the USPTO's interpretation of the PTA statute after the USPTO reduced the calculated "B" delay of the '622 patent by the number of days between the filing of an RCE and the issuance of the patent.
As shown in the following timeline, Exelixis filed the application that ultimately issued as the '622 patent on January 15, 2008. Thus, the three-year deadline for the USPTO to issue the patent fell on January 15, 2011. The USPTO mailed a final rejection on March 9, 2011. In response, Exelixis filed an RCE along with a substantive amendment on April 11, 2011. The '622 patent ultimately issued on August 2, 2011, with a total PTA of 368 days.
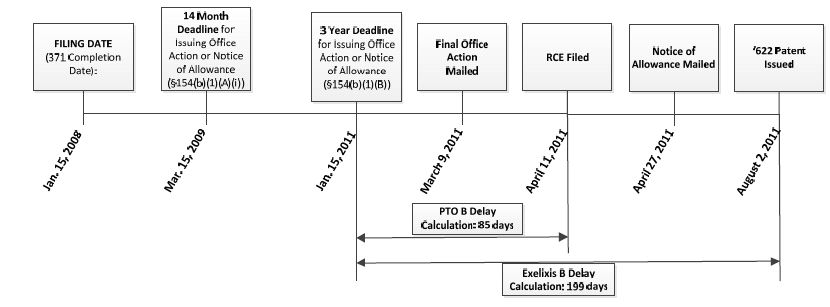
Exelixis did not dispute the USPTO's calculation of "A" delay (344 days), "C" delay (0 days), or reduction due to Applicant delay (61 days), but rather disputed the USPTO's "B" delay calculation of 85 days. Pursuant to the USPTO's traditional interpretation of Section 154(b)(1)(B)(i), the USPTO calculated the "B" delay as the difference between (1) the total number of days (199) from the day after the three-year deadline (January 15, 2011) to the issue date (August 2, 2011), and (2) the total number of days (114) between the filing of the RCE (April 11, 2011) and the issue date.
In contrast, Exelixis argued that the USPTO's interpretation was contrary to the plain language of the statute and that they are entitled to the full 199 days between the three-year deadline and the issue date because the RCE was filed after the three-year deadline.
The district court agreed with Exelixis that the language of the PTA statute is clear and unambiguous and that the USPTO's interpretation was erroneous. Applying statutory construction principles and reasoning that the statute does not treat an RCE filing as applicant delay, the district court stated that the statute's "plain language neither addresses nor requires that an applicant's PTA be reduced by the time required to process an RCE that is filed after the expiration of the three year period." The court further stated that "subparagraph (B) clearly provides no basis for any RCE's to reduce PTA; instead, RCE's operate only to toll the three year guarantee deadline, if, and only if, they are filed within three years of the application filing date." Thus, the district court held that Exelixis' RCE had no impact on PTA since it was filed after the three-year deadline had passed. Accordingly, Exelixis is entitled to the full 199 days of "B" delay.
The district court dismissed the USPTO's arguments that the ruling could result in disparate treatment of applicants, responding that the USPTO itself can solve any perceived problems by ensuring that applications are timely processed within the three-year window. It remains to be seen whether the USPTO will appeal the decision.
Considerations for Patentees and Applicants in Light of the Exelixis Decision
According to the district court, "once the three year clock has run, PTA is to be awarded on a day for day basis regardless of subsequent events" and therefore an RCE has no impact on PTA when filed after the three-year deadline has passed. Thus, the PTO can no longer deduct time for an RCE filed after the three-year deadline. However, an RCE filed before the three-year deadline tolls "B" delay.
The implications of this ruling could be significant for patent terms, particularly in the areas of biotechnology and high tech since pendency of applications in these areas typically exceed three years and since loss of any potential additional term can often mean millions of dollars in lost revenue. Some commentators have estimated that this ruling could impact approximately 10% of recently issued patents. Depending on the patent, the effects of the decision could amount to additional PTA of days, weeks, or in some cases, even years.
Given the large number of recently issued and soon-to-be issued patents that could be impacted, it is likely that the USPTO will appeal to the Federal Circuit. Thus, the finality of the district court's interpretation of the statute will remain delayed until that review is complete. In the interim, patentees and applicants should consider taking a proactive approach to assess the decisions' impact on their recently issued patents, as well as allowed and pending applications.
Pending Applications
For pending applications, in light of Exelixis, Inc. v. Kappos, Applicants and their patent attorneys should be cognizant of the impact on potential PTA when considering filing an RCE, particularly in those applications that have been pending for less than three years at the time the applications become ripe for an RCE.
Allowed Applications
Applicants and their patent attorneys should consider identifying any allowed application in which an RCE was filed after the three-year deadline, which triggers "B" delay. Since "B" delay is not calculated until the projected issue date is identified on the Issue Notification, applicants and their patent attorneys should verify the correctness of the PTA for these identified applications as stated on the issue notification and, ultimately, the face of the patent. In the meantime, while we all wait to see if the USPTO will appeal the decision, it may be worthwhile in some instances to file a PTA petition.
Granted Patents
Patents issued within 180 days of the November 1, 2012 decision may be eligible for a recalculation of PTA in light of Exelixis, Inc. v. Kappos. See 35 U.S.C. § 154(b)(4)(A). Depending on how much time has passed since the issuance of the patent, such a recalculation may require petitioning the USPTO and/or filing a lawsuit in the U.S. District Court for the Eastern District of Virginia. See id. While waiting to see if the USPTO will appeal and depending on the patent's importance, patentees should consider whether pursuing such a recalculation in the USPTO or the court is worthwhile.
Additional Challenges to USPTO's Interpretation of Section 154(b)(1)(B)
Several other cases challenging the USPTO's interpretation of Section 154(b)(1)(B) are also pending before the Eastern District of Virginia including Biogen Idec MA Inc. v. Kappos (Civil Action No. 1:12-cv-01171), filed October 19, 2012, and Human Genome Sciences, Inc. v. Kappos (Civil Action No. 1:12-cv-00607), filed June 1, 2012. These cases are challenging the USPTO's interpretation that "any time consumed by continued examination" includes any time between mailing of the notice of allowance and the issue date. Venable's patent attorneys continue to monitor these cases.
Possible Additional Implications of Exelixis, Inc. v. Kappos
Other exceptions to the guarantee of no more than three-year application pendency provided for in Section 154(b)(1)(B), include the exclusion of any time an application spends involved in an interference under Section 135(a), any time an application is subject to a secrecy order under Section 181, and any time spent in appellate review at the Patent Office or by a Federal Court. 35 U.S.C. § 154(b)(1)(B)(ii). While these delays are explicitly accounted for as "C" delay, based on similarities in statutory constructions, it appears that the reasoning in Exelixis may be equally applicable to these exclusions.
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.



