The FTC flexed its new-found civil penalty muscle last week by filing the first case pursuant to the COVID-19 Consumer Protection Act, which gives the FTC authority to seek civil penalties for deceptive COVID-related acts and practices. ICYMI, see our blog post about the civil penalty authority here.
Ordinarily, the FTC is only authorized to seek penalties for violating a prior Court or Commission order, cease and desist order or trade regulation. This new power extends "[f]or the duration of a public health emergency," and permits the FTC to seek penalties for deceptive acts or practices in or affecting commerce that is associated with (1) the treatment, cure, prevention, mitigation, or diagnosis of COVID-19, or (2) a government benefit related to COVID-19.
The FTC's first action under the COVID-19 Consumer Protection Act, U.S. v. Quickwork LLC and Eric Anthony Nepute, is filed in the United States District Court for the Eastern District of Missouri. The complaint alleges that, despite prior receipt of a letter warning or unsubstantiated COVID-19 efficacy claims, Nepute (a chiropractor) and his company Quickwork deceptively marketed vitamin D and zinc products under the "Wellness Warrior" brand for the treatment, prevention, and cure of COVID-19. The Complaint contains excerpts from the defendants' advertisements, marketing
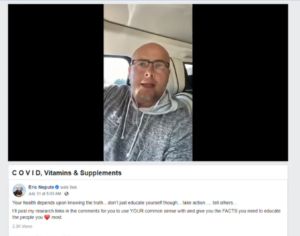 emails, videos,
and social media posts suggesting that use of Wellness Warrior
products will, among other things, (1) treat or prevent COVID-19;
(2) decrease the chance of contracting COVID-19; and (3) decrease
the chance of death upon diagnosis with COVID-19. The Complaint
also references advertisements stating that the Wellness Warrior
products provide equal or better protection than currently
available COVID-19 vaccines and that the defendants' various
representations relating to the efficacy of Wellness Warrior
products are scientifically proven. Despite these representations,
the Complaint alleges that there are no published studies, or other
competent and reliable scientific evidence, supporting the efficacy
of vitamin D3, zinc, or the Wellness Warrior products in treating
or preventing COVID-19.
emails, videos,
and social media posts suggesting that use of Wellness Warrior
products will, among other things, (1) treat or prevent COVID-19;
(2) decrease the chance of contracting COVID-19; and (3) decrease
the chance of death upon diagnosis with COVID-19. The Complaint
also references advertisements stating that the Wellness Warrior
products provide equal or better protection than currently
available COVID-19 vaccines and that the defendants' various
representations relating to the efficacy of Wellness Warrior
products are scientifically proven. Despite these representations,
the Complaint alleges that there are no published studies, or other
competent and reliable scientific evidence, supporting the efficacy
of vitamin D3, zinc, or the Wellness Warrior products in treating
or preventing COVID-19.
The Complaint asserts ten causes of action for violations of the FTC Act and the COVID-19 Consumer Protection Act, and seeks preliminary and permanent injunctive relief, rescission or reformation of contracts, the refund of monies paid, restitution, the disgorgement of ill-gotten gains, civil penalties and costs. With respect to civil penalties, the Complaint alleges that each dissemination of an allegedly-deceptive advertisement constitutes a separate violation for purposes of calculating monetary civil penalties," and that the Court is authorized to award penalties up to $43,792 for each such violation.
In addition to the remedies sought under the COVID-19 Consumer Protection Act, the FTC is also seeking refunds, restitution and disgorgement under the FTC Act despite the current uncertainty regarding whether the FTC can pursue monetary remedies at all as part of a request for injunctive (equitable) relief. The Supreme Court is poised to rule on that issue very shortly in AMG Capital Management, LLC v. Federal Trade Commission, No. 19-508 (U.S.).
Regardless, the FTC's authority to seek civil penalties for allegedly deceptive COVID-19 advertising is clear and we should expect that this will not be the only instance in which the agency seeks to use its new authority.
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.




