The Rights and Obligations of the new Medicine and Medical Devices Regulatory Authority
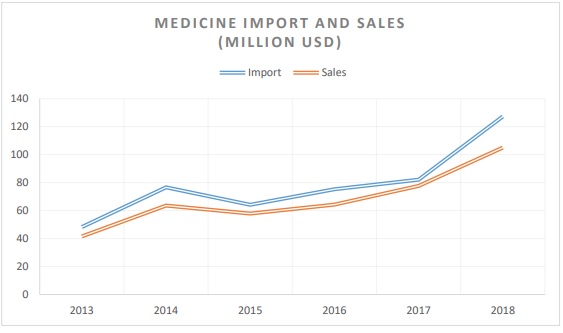
Until December 2020, the Ministry of Health, the Centre for Health Development and the relevant departments of the General Agency of Specialized Inspection were responsible for the regulation with regards to import, export and distribution of medicines and medical devices under their respective functions. According to a study in the pharmaceutical sector in 2018, Mongolia imported medicines amount of 362.5 billion tugriks (127.2 million USD) and sold the amount of 299 billion tugriks (105 million USD) (wholesale price), which is respectively increased by the amount of 128.4 billion tugriks (45 million USD) in import of medicines and grow in the amount of 77,8 billion tugriks (27.2 million USD) in medicine sales compared to 2017. 1
According to an independent study conducted by BioMed Central Public Health in 2020, when samples of 1770 medicine products were taken from Ulaanbaatar and 4 provinces, the 179 samples equal to 10.1% did not meet the requirements of the standard and 76 samples equal to 4.3% of the total amount of the samples were not registered in Mongolia2
The Government of Mongolia established a new agency, which named as Medicine and Medical Devices Regulatory Authority in accordance with the Decree No 222 of the Government of Mongolia 3 dated 16 December 2020 in order to overcome the existing inconsistent and inefficient structure to make the state regulation and supervision in the pharmaceutical market. The newly established Medicine and Medical Devices Regulatory Authority shall exercise and fulfill the following rights and obligations for the importing and distribution of medicines and medical devices.
1. Import of medicines and medical devices
The agency for procurement of medicines shall get the license for importing medicines and medical devices from the Medicine and Medical Devices Regulatory Authority to import and export medicines and medical devices.
The import license shall specify names, types, doses and quantities of medicines and medical devices, names of manufacturers, frontier crossing-point and the date.
|
Medicine and Medical Regulatory Authority shall issue an import license for the following medicines and medical devices. |
|
|
Prohibited items on import and export of medicines and medical devices. |
|
In accordance with the list approved by the Government, the Medicine and Medical Devices Regulatory Authority may concede a right to directly supply immunization products, medicines and medical devices through import, on the basis of concluding a direct contract with internationally recognized medicine manufacturers and suppliers.
2. Distribution of medicines and medical devices
The Medicine and Medical Devices Regulatory Authority shall control the regulation to issue the study of medicine quality, price surveillance, sales licenses, and marketing authorization set forth by law.
- Issues a registration of medicines and bioactive products;
- Issues a market research and surveillance on quality and safety of medicines and bioactive products
- Issues a special license to manufacture and sales;
- Issues a quality assurance;
- Issues a Certificate of satisfying the pharmaceutical industry standards of GMP;
- Issues a specialized inspection on pharmacological activities;
- Issues a control over side-effects of medicine;
- Issues marketing authorization and control of medicines and bioactive products;
- Monitoring and evaluation the assortment and prices of vital and essential medicines and other related matters on medicines and medical devices.4
By establishing the new Medicine and Medical Devices Regulatory Authority, the activities related to the manufacture, import, export, sales, distribution, and control of medicine, medical devices, and bioactive products have been unified by the government.
Footnotes
1 http://hdc.gov.mn/media/files/2018_Kedu7Vn.pdf
2 https://bmcpublichealth.biomedcentral.com/articles/10.1186/s12889-020-08897-x
3 https://www.legalinfo.mn/law/details/15843?lawid=15843
4 https://www.legalinfo.mn/law/details/85?lawid=85
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.

