- within Litigation and Mediation & Arbitration topic(s)
- with readers working within the Law Firm industries
I. INTRODUCTION
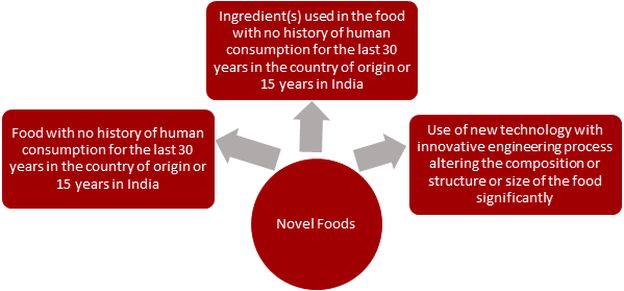
Increased connectivity around the world has started a new wave in the market for novel foods. Novel foods such as genetically modified foods and food products, cell-based meat, eggs, and dairy alternatives, as well as plant-based and fermentation-derived proteins, have entered the Indian market. Therefore, it becomes important to understand the regulatory framework put in place for novel foods. Under the Food Safety and Standards (Health Supplements, Nutraceuticals, Food for Special Dietary Use, Food for Special Medical Purposes, Functional Food, and Novel Food) Regulations, 2016 ("2016 Regulations"), novel foods are defined as:
In the Indian plant-based food market, several players like Good Dot and One Good feature a wide range of novel food products including soy milk, coconut milk, almond cheese, and plant-based meat alternatives, among others. In addition, an Indian start-up, Clear Meat, has successfully developed and tested its first cultivated chicken mince, with plans to launch its market-ready product by 2023. Myoworks also aims to manufacture ingredients and scaffolds for the cultivated-meat industry and has received INR 50 lakhs from the Department of Biotechnology, India to demonstrate a preliminary proof of concept.
Plant-based alternatives are gaining popularity worldwide with start-ups like Zero Egg and Vly Foods offering a multitude of products. Besides, Eat Just, a Californian company, made history in December 2020, when its cultivated chicken became the first cultured meat product to receive regulatory approval in Singapore.
II. REGULATIONS GOVERNING NOVEL FOODS IN INDIA
The Food Safety and Standards Authority of India ("FSSAI"), on 11 September 2017, notified the Food Safety and Standards (Approval of Non-Specified Food and Food Ingredients) Regulations, 2017 ("2017 Regulations"). Under the 2017 Regulations, prior approval is required for certain articles of food or food ingredients, before its introduction in the market.
In addition, the 2016 Regulations govern eight categories of foods including novel foods. The regulations provide a list of ingredients and additives that are allowed to be used in specified food categories. The Food Business Operators ("FBO") intending to manufacture, import or sell the specified foods are required to adhere strictly to these regulations.
Although in March 2022, the Food Safety and Standards (Health Supplements, Nutraceuticals, Food for Special Dietary Use, Food for Special Medical Purpose, and Prebiotic and Probiotic Food) Regulations, 2022 ("2022 Regulations") was enacted, superseding the 2016 Regulations, the 2022 Regulations specifically excludes novel foods from the applicability of the regulations and therefore the 2016 Regulations and 2017 Regulations continue to govern novel foods.
Recently, in 2022, the FSSAI notified the Food Safety and Standards (Vegan Foods) Regulations, 2022 ("Vegan Regulations") which govern plant-based food products which are novel foods. The Vegan Regulations stipulate the definition of vegan, requirements as a part of the prior-approval process and labelling declarations, among others.
III. License for Novel Food Manufacturers
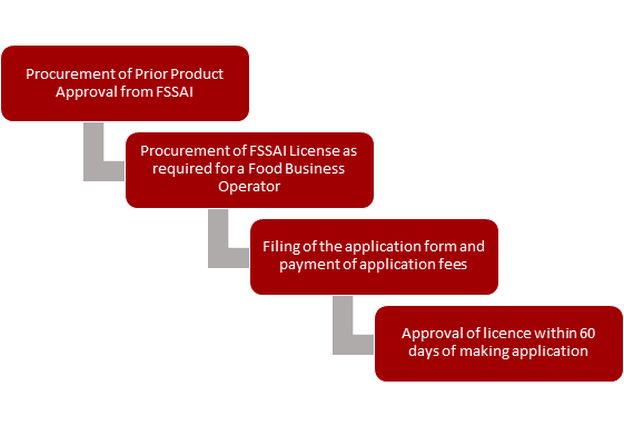
Novel food businesses are required to obtain a central license under the Food Safety and Standards (Licensing and Registration of Food Business) Regulations, 2011 ("2011 Regulations") from the licensing authorities, whereas petty FBOs involved in manufacturing of novel foods are only required to register themselves under regulations. In addition to the specific procedures for license application, FBOs are required to comply with hygienic and safety practices. The 2011 Regulations also necessitate all renewals and changes in the business to be appropriately communicated and approved by the licensing authority. A novel food manufacturer or importer needs prior approval from FSSAI as per the 2017 Regulations before applying for a central licence.
IV. Process to procure the Novel Food Licence
The process for obtaining a license for the manufacture of novel foods under the 2011 Regulations is:
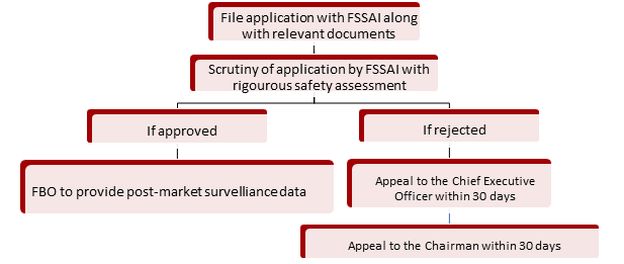
V. Prior Product Approval Process from FSSAI for Novel Foods
VI. Conclusion
Plant-based foods, such as burgers made from plant-based meat, are gaining global popularity due to their eco-friendly nature. Indian companies are also developing innovative plant-based dairy products that cater to consumers' tastes and dietary needs, particularly those with lactose intolerance. However, the current legal regime for novel foods in India is fraught with challenges. The FSSAI has wide discretion in the regulatory process at various steps. For example, FSSAI has the discretion to provide extra steps of assessments such as safety assessments like clinical trials on the Indian population. There are no specific guidelines for these clinical trials as of now. Further, the requirement of post-market surveillance is also specified by FSSAI on a case to case basis with no guidelines for FSSAI to determine the need for post-market surveillance. Moreover, no specific timeline is defined in the 2017 Regulations for the prior approval of novel foods, leading to delays for FBOs. Thus, these wide discretions of FSSAI create uncertainty and make the regulatory mechanism cumbersome.
Novel foods are still facing challenges due to their high cost which restricts their accessibility and consumer acceptance. However, ongoing research aims to make them healthier and more desirable alternatives to conventional foods.
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.




