The amended Patent Law came into effect on June 1, 2021, in which the new added Article 70, paragraph 1 endows the National Intellectual Property Administration of China (CNIPA) with the authority to solve patent infringement disputes which have a significant impact nationwide upon the request of the patentee or the interested person.
In accordance with it, CNIPA has promulgated the Administrative Adjudication Measures for Significant Patent Infringement Disputes (hereinafter referred to as the "Adjudication Measures"), which also came into effect on June 1, 2021. Article 22 of the "Adjudication Measures" stipulates that: "When CNIPA solves a patent infringement dispute, it shall make a decision within three months from the date when the request is accepted. If the case cannot be closed within the specified time limit due to the complexity of the case or other reasons, the time limit may be extended for one month upon approval." Because the administrative adjudication of patent infringement disputes has the characteristics of high efficiency, low cost, strong specialization, and simple procedures, and it is conducive to the rapid resolution of patent infringement disputes and plays the role of "diverting valve" for resolving civil disputes, it has received wide attention.
Under this circumstance, the CNIPA accepted the first batch of two requests for the administrative adjudication of significant patent infringement disputes on November 5, 2021. Under the concern of all sectors of society, the CNIPA made corresponding decisions on these two cases on July 27, 2022 (Guo Zhi Bao Cai Zi [2021] No. 1 and Guo Zhi Bao Cai Zi [2021] No. 2).
I. SUMMARY OF THE CASES
The two cases all relate to the patent for invention No. ZL201510299950.3 with the title of "8-[3-AMINO-PIPERIDIN-1-YL]-XANTHINES, THE PRODUCTION THEREOF AND THE USE OF THE SAME AS MEDICAMENTS" (hereinafter referred to as the "patent of interest"). The requestor is BOEHRINGER INGELHEIM PHARMA GMBH & CO. KG, the patentee of the patent of interest. The respondents are Guangdong HEC Pharmaceutical Co., Ltd. (hereinafter referred to as the "Guangdong HEC") and Yichang HEC Changjiang Pharmaceutical Co., Ltd. (hereinafter referred to as the "Yichang HEC"), respectively.
The requestor asserts that: (I) The patent of interest is currently in a valid state. (II) The patent of interest seeks protection of a compound having a generic name linagliptin, a composition comprising linagliptin or a salt thereof, and use of linagliptin or a salt thereof in the preparation of a composition for the treatment of DPP-IV related disorders (e.g., type 2 diabetes). (III) Guangdong HEC filed a request for marketing license of the generic drug linagliptin tablet (hereinafter referred to as the "alleged infringing product") before the National Medical Products Administration on July 4, 2018, which was approved on July 8, 2020. From February 2021, Guangdong HEC publicized the alleged infringing product on the drug procurement platforms in many provinces (autonomous regions or municipalities) including Shanghai, Guangdong, Jiangxi, Hainan, Gansu, Sha'anxi, Henan, Fujian and Shandong, and offered for sale the alleged infringing products to medical institutions in the region. (IV) The two respondents are associated companies, and jointly engaged in the R & D, production, sales and distribution of generic drugs. The technology, marketing license and sales rights of 27 products including the alleged infringing product were assigned by Guangdong HEC to Yichang HEC, the latter being the actual production, sales and beneficiary entity of the alleged infringing product. As the procedure of transferring the drug marketing license has not been completed, Yichang HEC still entrusts Guangdong HEC to produce the alleged infringing product and publicize the alleged infringing product on the drug procurement platforms in the name of Guangdong HEC. (5) The alleged infringing product falls within the protection scope of the patent of interest. Accordingly, the requestor submitted a request for administrative adjudication of significant patent infringement disputes before the CNIPA.
The petitions of the requestor are: (I) ordering the respondents to immediately stop the manufacturing, selling and offering for sale the alleged infringing product throughout the country; and (II) ordering the respondents to immediately withdraw the applications for publicization of the alleged infringing product from the drug procurement platforms where they have applied for publicization (including but not limited to Shanghai, Fujian, Shandong, Guangdong, Jiangxi, Hainan, Gansu, Sha'anxi, and Henan) that have applied for online connection.
The respondents argued that: (I) The patent of interest is a divisional application of patent No. ZL03819760.X, based on which the requestor has filed a lawsuit before Shanghai Intellectual Property Court. The prerequisites for requesting administrative adjudication of significant patent infringement disputes include that "neither party has filed a lawsuit before the people's court"; accordingly, the current request for administrative adjudication does not meet the requirements for accepting the request. (II) The publicization of alleged infringing product by Guangdong HEC on the drug procurement platforms in various regions belongs to the exceptions of infringement specified in the Patent Law and does not constitute offering for sale.
The CNIPA accepted the request on November 5, 2021, and organized a five-member panel to examine the two cases. On December 10, 2021, the respondents applied for suspension of the processing of the cases on the ground that a request for invalidation has been filed and accepted. The CNIPA agreed to suspend the processing on December 15, 2021. After the invalidation case was closed due to the withdrawal of the request by the requestor for invalidation, the present cases were resumed on May 16, 2022. On May 18, 2022, the respondents applied again for suspension of the processing of the cases on the ground that another request for invalidation has been filed and accepted. Upon discussion, the panel decided not to suspend the processing of the cases again, and to examine the two cases in combination. After an oral hearing, the CNIPA made a decision on July 27, 2022.
II. FACTS AFFIRMED BY THE PANEL
Upon the investigation of facts, the panel affirmed the following facts:
- The patent of interest is currently in a valid state.
- The National Medical Products Administration approved on July 8, 2020 the request for marketing license of linagliptin tablet filed by Guangdong HEC. The main ingredient thereof is linagliptin; the indication is type 2 diabetes, and the license holder and manufacturer are both Guangdong HEC.
- The respondent manufactured the alleged infringing product and actually sold the same in at least Shanghai, Guangdong and Jiangxi.
- The alleged infringing product has been publicized or executed on the relevant official websites in many provinces (autonomous regions or municipalities) including Shanghai, Guangdong, Jiangxi, Sha'anxi, Inner Mongolia, Xinjiang, Guangxi, Jiangsu, Hunan, Hubei, Heilongjiang and Zhejiang; and has been at least publicized on the relevant official websites in many provinces (autonomous regions or municipalities) including Fujian, Shandong, Hainan, Gansu, Henan, Hebei, Ningxia, Yunnan, Guizhou, Qinghai, Sichuan and Beijing.
III. MAIN DISPUTES
In the decisions, the following main disputes were solved.
(I) In respect of whether the request for administrative adjudication filed by the requestor meets the requirements for accepting a request for administrative adjudication of significant patent infringement dispute
Article 3 of the "Adjudication Measures" stipulates that: "Either one of the following situations belong to significant patent infringement disputes: ... (3) significant cases across provincial administrative regions; ...".
The panel decides that the alleged infringing product has been sold in Shanghai, Guangdong and Jiangxi, and publicized or executed in 24 provinces (autonomous regions or municipalities) such as Shanghai, which belongs to the "significant cases across provincial administrative regions" as stipulated in Article 3 of the "Adjudication Measures".
In respect of the arguments of the respondents that the current request for administrative adjudication does not meet the requirements for accepting the request for administrative adjudication of significant patent infringement dispute since the requestor has filed a lawsuit before the people's court, Article 4 of the "Adjudication Measures" stipulates that: "The request for administrative adjudication of significant patent infringement dispute shall comply with Article 3 and meet the following requirements: ... (4) the people's court has not placed said patent infringement dispute on file".
The panel decides that the parent application and the divisional application have different protection scopes. The lawsuit filed before the people's court in respect of the parent application and the request for administrative adjudication in respect of the divisional application relate to different evidence, facts and reasons. They are not the same patent infringement dispute. The requestor did not file a lawsuit before the people's court in respect of the patent of interest.
In view of the above, the panel decides that the acceptance of the present cases comply with the "Adjudication Measures".
(II) In respect of whether the processing of the present cases should be suspended again
Article 17 of the "Adjudication Measures" stipulates that: "Under one of the following circumstances, a party may request for suspension of the processing of the case, or the CNIPA may decides to suspend the processing of the case: ... (6) the case shall be decided on the basis of the examination results of another case, whereas said another case has not been decided yet; ...".
In respect of the second request for suspension filed by the respondents, the panel decides that the patent of interest is a patent for invention, which was granted after a substantive examination. Referring to Article 11 of Several Rules of the Supreme People's Court on the Application of Laws When Hearing Patent Dispute Cases, in the patent infringement dispute cases heard by the People's court, where the defendant files a request for invalidation during course of replying, the People's court may not suspend the litigation.
Meanwhile, in the present case, the same requestor for invalidation has filed a previous requestor for invalidation, whereas the patent is still valid after the invalidation procedure. By generally considering the justice and the efficiency of the administrative adjudication of patent infringement disputes, the panel decides not to suspend the processing of the cases again.
(III) In respect of whether the alleged infringing product falls within the protection scope of the patent of interest
The panel affirms that the description of the alleged infringing product recites that the generic name of the drug is linagliptin tablet, and the main ingredient is linagliptin, which has a chemical name of 8-[(3R)-3-amino-1-piperidinyl]-7-(2-butyn-1-yl)-3,7-dihydro-3-methyl-1-[(4-methyl-2-quinazolinyl)methyl]-1H-purine-2,6-dione, and a chemical structure as follows:
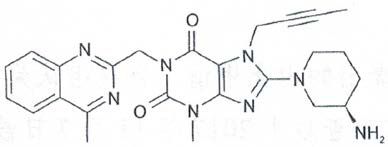
The molecular formula is C25H28N8O2, and the indication is "type 2 diabetes".
Claims 1, 2, 5 and 6 of the patent of interest protect the following general formula substituted by certain groups, or enantiomers, diastereomers, mixtures thereof, or salts thereof:
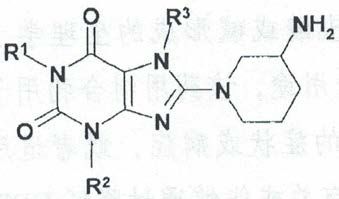
The panel asserts that for a specific compound, as long as the core structure and the substituents on the core structure of said specific compound all fall within the ranges of various alternatives listed for the general formula, it can be determined that said specific compound falls within the protection scope of the general formula. Based on this, the main ingredient of the alleged infringing product, linagliptin, falls within the protection scope of claims 1, 2, 5 and 6 of the patent of interest. Meanwhile, as a drug for treating type 2 diabetes, it also falls within the protection scope of the pharmaceutical composition claims and pharmaceutical use claims of the patent of interest. In fact, the only compound defined in the use claim 43 of the patent of interest is exactly linagliptin.
(IV) In respect of whether the respondents conducted manufacturing, selling and offering for sale of the alleged infringing product
Manufacturing and selling. The panel asserts that the alleged infringing product has been purchased in various medical institutions in many places such as Shanghai, Guangdong and Jiangxi. In other words, the alleged infringing product has been manufactured and actually sold. The outer package and the description of the sold alleged infringing product both specifically indicate that the license holder and manufacturer are both Guangdong HEC. Therefore, Guangdong HEC conducted manufacturing and selling of the alleged infringing product.
Offering for sale. The panel asserts that publicization of a drug is a necessary conduct for a drug manufacturer to participate in centralized procurement through a provincial drug centralized procurement platform. Through this conduct, a manifestation of selling the drug is made to corresponding medical institutions, and the drug manufacturer that wins the bidding on the drug centralized procurement platform have the obligation to ensure the supply of drug. The respondent made a clear manifestation to sell the alleged infringing product to medical institutions in the province (autonomous region or municipality) where the centralized drug procurement platform is located, and has even conducted selling in various places including Shanghai, Guangdong and Jiangxi. Therefore, Guangdong HEC conducted offering for sale of the alleged infringing product.
In respect of whether the publicization of the drug belongs to the exceptions of infringement. Article 75, Item (5), of the Chinese Patent Law stipulates that "none of the following shall be deemed as infringement of the patent right: ... (5) where for the purpose of providing information needed for the regulatory examination and approval, any person manufactures, uses or imports a patented medicine or a patented medical device, and where any person makes, imports the patented medicine or the patented medical device exclusively for such person". The panel decides that, firstly, the conducts specifically listed in Article 75, Item (5), of the Chinese Patent Law are manufacturing, using and importing, not including offering for sale; secondly, in the present case, the respondent has completed the regulatory examination. Therefore, the publicization of the drug by the respondent does not belong to the exceptions of infringement stipulated in Article 75, Item (5), of the Chinese Patent Law.
IV. DECISIONS OF ADMINISTRATIVE ADJUDICATION
In view of the above, the panel decides that under
the circumstance that the patent owned by the requestor is still valid, the respondent, without the authorization of the requestor, manufactured, sold and offered for sale linagliptin tablets for production or business purposes, which constitutes an infringement of the requestor's patent right. Accordingly, the following administrative adjudications are made:
- ordering the respondents to immediately stop the manufacturing, selling and offering for sale the product infringing the patent right (patent No. ZL201510299950.3) of the requestor, BOEHRINGER INGELHEIM PHARMA GMBH & CO. KG; and
- ordering the respondent Guangdong HEC Pharmaceutical Co., Ltd. to immediately withdraw the publicization of linagliptin tablet from the drug procurement platforms.
V. DISCUSSION
The decision of the present cases provides an example in both the procedural and substantive aspects for the administrative adjudication of significant patent infringement disputes at the national level in China, and marks the gradual improvement of the system.
It should be noted that the decisions made by CNIPA are also subject to judicial review, that is, the above decisions are not final. If any party is not satisfied with the above decisions, they may file an administrative lawsuit before the Beijing Intellectual Property Court.
According to the statistics of the International Diabetes Federation, there are about 100 million patients with diabetes in China, and among the drugs for type 2 diabetes, DPP-4 inhibitors account for 33% of the non-insulin drugs used globally. With its unique metabolic advantages, linagliptin ranks second among all DPP-4 inhibitors in terms of global sales volume and has a good market prospect. As HEC Pharmaceutical Group is the first generic enterprise of linagliptin, the results of the above administrative adjudications and the trial trend of related disputes may affect the market pattern of the generic drug linagliptin in the future.
It should be noted that in respect of the decision of the panel not to suspend the processing of the cases, Article 18 of the "Adjudication Measures" stipulates that "under any of the following circumstances, the CNIPA may not suspend the processing of the case: (1) where the search report or patent evaluation report provided by the requestor does not show any defect based on which the patent for utility model or design patent shall not be granted; (2) where a decision of an invalidation procedure has maintained the validity of the patent for utility model or design; (3) the reasons for suspension submitted by the relevant party are obviously untenable". That is, it does not provide that the case of dispute over a patent for invention may not be suspended. We have to see that this is a flaw in Article 18 of the "Adjudication Measures".
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.

