On November 13, 2017, the European Commission (EC) published its roadmap (Roadmap) for the implementation of Regulation (EU) 2017/745 on Medical Devices (MDR).
The MDR entered into force on May 26, 2017, but most of its provisions will only apply as of May 26, 2020, and several key aspects of the new European medical device system still require further implementation by the EC. Overall, it seems that the MDR will not become operational anytime soon despite the industry being encouraged to be compliant therewith as soon as possible.
This Legal Update highlights the key dates for the MDR and explains the Roadmap. It does not cover Regulation (EU) 2017/746 on In Vitro Diagnostic Medical Devices although IVD devices are also covered by the Roadmap.
Key Dates for the Medical Devices Regulation (MDR)
The MDR replaces Directive 93/42/EEC on medical devices (MDD) and Directive 90/385/EEC on active implantable medical devices (AIMDD).
May 5, 2017: Publication of the MDR in the
Official Journal of the European Union.
May 26, 2017: Entry into force of the MDR.
November 26, 2017:
- Notified Bodies (NBs) may submit an application for designation under the MDR.
- Designation of the national authorities responsible for the implementation of the MDR and establishment of the Medical Device Coordination Group (MDCG)
May 26, 2018: Cooperation of the National Competent Authorities (NCA) for the uniform implementation of the MDR.
February 25, 2020: Adoption of national penalties for infringements of the MDR.
March 25, 2020: Operation of Eudamed, the European database of medical devices.
May 26, 2020: Application of the MDR.
- Medical devices that do not comply with the MDR may no longer be placed on the market.
- Reporting of serious adverse events and device deficiencies in accordance with the MDR for all clinical investigations, including those started before the application of the MDR.
- Adoption of common specifications for single- use devices and their reprocessing.
- NBs that are not designated and notified in accordance with the MDR may no longer carry out conformity assessment procedures and issue certificates.
May 26, 2021: Unique Device Identification (UDI) carriers must be placed on implantable devices and class III devices.
May 27, 2022: Certificates of conformity issued by NBs in accordance with Annex 4 to the AIMDD or Annex IV to the MDD before May 25, 2017, become void.
May 26, 2023: UDI carriers must be placed on class IIa and class IIb devices.
May 27, 2024: Certificates of conformity issued by NBs in accordance with the AIMDD or the MDD from May 25, 2017, become void.
May 26, 2025: UDI carriers must be placed on class I devices.
May 27, 2025: Devices lawfully placed on the market under the AIMDD and MDD may no longer be made available on the market or put into service.
May 27, 2027: Application of the coordinated assessment procedure for clinical investigations under the MDR.
Roadmap for Implementation
The Roadmap describes the next steps and actions to undertake for implementing the MDR.
Specific provisions of the MDR empower the EC to take implementing actions, but guidance documents have to be adopted beforehand in order to ensure a harmonized interpretation of the legislation. The Roadmap does not detail the content of the EC's implementing measures and therefore does not inform the industry about the actions to be taken in order to comply with the MDR. Rather, it outlines how the different working parties may contribute to the development of those measures, i.e., may develop the documents and structures on which to build the implementation. The Roadmap is more a pre- roadmap than a roadmap as most of the actions described in it involve developing guidance documents, clarifications and best practices to implement the medical devices rules.
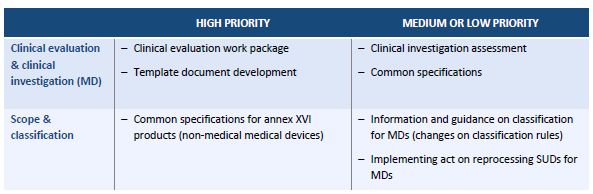
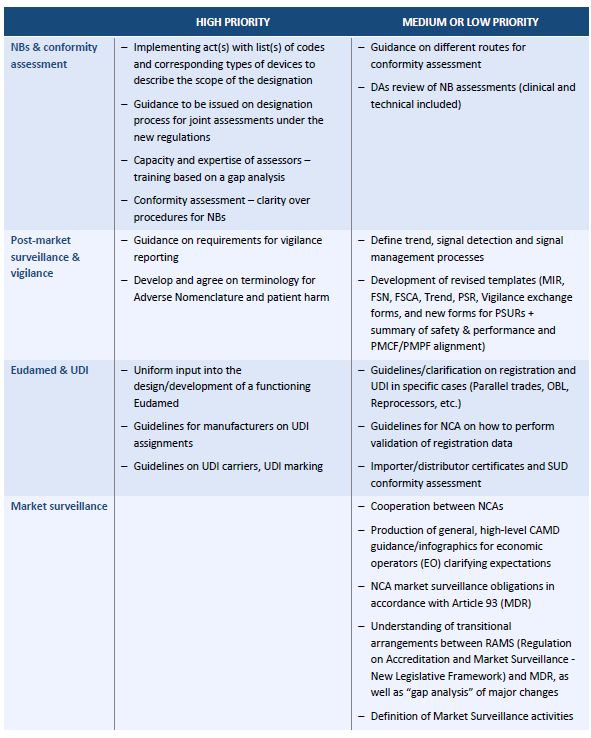
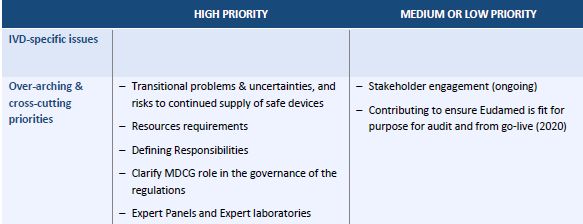
The Roadmap focuses on eight technical areas. For each area, it lists activities, including a brief description, their priority level and the parties responsible for carrying them out. Overall, the Roadmap envisages 35 activities in relation to medical devices (MDs), of which 17 are classified as "high priority." It is remarkable that the package "market surveillance" has no "high priority" item.
Visit us at mayerbrown.com
Mayer Brown is a global legal services provider comprising legal practices that are separate entities (the "Mayer Brown Practices"). The Mayer Brown Practices are: Mayer Brown LLP and Mayer Brown Europe – Brussels LLP, both limited liability partnerships established in Illinois USA; Mayer Brown International LLP, a limited liability partnership incorporated in England and Wales (authorized and regulated by the Solicitors Regulation Authority and registered in England and Wales number OC 303359); Mayer Brown, a SELAS established in France; Mayer Brown JSM, a Hong Kong partnership and its associated entities in Asia; and Tauil & Chequer Advogados, a Brazilian law partnership with which Mayer Brown is associated. "Mayer Brown" and the Mayer Brown logo are the trademarks of the Mayer Brown Practices in their respective jurisdictions.
© Copyright 2017. The Mayer Brown Practices. All rights reserved.
This Mayer Brown article provides information and comments on legal issues and developments of interest. The foregoing is not a comprehensive treatment of the subject matter covered and is not intended to provide legal advice. Readers should seek specific legal advice before taking any action with respect to the matters discussed herein.

