In Neptune Generics, LLC v. Auspex Pharmaceuticals, Inc., IPR2015-01313, Paper No. 25 (PTAB Dec. 9, 2015) ("Neptune"), the Patent Trial and Appeal Board ("the Board") issued an opinion denying institution of inter partes review of U.S. Patent No. 7,456,317 B2 ("the '317 patent"). The '317 patent claims an analog of venlafaxine (Effexor®) in which nine carbon-hydrogen (C-H) bonds are replaced with carbon-deuterium (C-D) bonds. ('317 patent, col. 5:61-67) Claim 1 is representative:
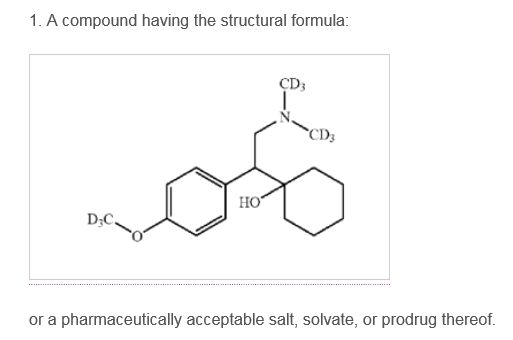
Venlafaxine's mechanism of action and metabolism have been studied extensively (id. at col. 4:6-19), and both venlafaxine and its active metabolite have half-lives that are much shorter than that "regarded as ideal for this class of compounds by most clinicians." (Id. at col. 4:41-50) Venlafaxine is a serotonin and norepinephrine reuptake inhibitor approved for the treatment of depression, generalized anxiety disorder, panic disorder, and social anxiety disorder. As the '317 patent acknowledges, "[d]euteration of pharmaceuticals to improve pharmacokinetics (PK), pharmacodynamics (PD), and toxicity profiles has been demonstrated previously with some classes of drugs." (Id. at col. 3:47-49)1
The '317 patent describes nearly 100 deuterated venlafaxine analogs, including the claimed one (id. at col. 10:51–col. 26:45), and states that enriching for deuterium alters the ratio of active venlafaxine metabolites, reduces unwanted metabolites, and increases the half-life of the parent drug and its metabolites. (Id. at col. 6:27-33)
The Petitioner challenged claim 1 of the '317 patent as being obvious over Fogelman2 in view of Miwa.3,4 The Petitioner primarily relied on Fogelman's disclosure that the major metabolites of venlafaxine are O-desmethylvenlafaxine ("ODV"), N-desmethylvenlafaxine ("NDV"), and N,O-didesmethylvenlafaxine ("N,O-DV"), depicted below.

(Neptune, Paper 25 at 9.) Fogelman states that "ODV has a receptor affinity profile similar to its parent compound [venlafaxine], while the latter two metabolites have little if any affinity for the . . . receptor sites." (Id. at 6) Fogelman further states that "approximately 90% of total intrinsic clearance was accounted for by the O-demethylation pathway," in which the venlafaxine methoxy group is converted to a hydroxy. (Id. at 7, 11)
The Petitioner relied on Miwa for the "teaching that for some drugs, isotope substitution (i.e., from hydrogen to deuterium) may reduce the rate of drug metabolism, and, therefore, may reduce the rate of clearance of the drug from the body." (Id. at 8) According to the Petitioner's expert, one of ordinary skill in the art would have been motivated to "deuterate the methyl groups involved in the primary metabolic pathways. . . . in order to reduce the rate of venlafaxine metabolism, reduce the clearance of venlafaxine from the body, and improve the duration of action and peak plasma levels of venlafaxine." (Neptune, Ex. 1002 at 10)
In its Preliminary Response,5 the Patent Owner relied on several references to support its argument that the '317 patent's claims are nonobvious over the cited prior art because it is not possible to predict whether any given isotope effect will provide a beneficial change in metabolism without extensive testing. (Neptune, Paper 25 at 10)
In particular, the Patent Owner presented evidence that a primary deuterium kinetic isotope effect "will only be observed if the breaking of the carbon-hydrogen bond is the rate-limiting step."6 (Id.) The Patent Owner also provided evidence that even if the breaking of the carbon-hydrogen bond is the rate-limiting step, the ordinary artisan would not have been able to predict the magnitude of the deuterium substitution effect.7 The Patent Owner pointed to Miwa – cited by the Petitioner – for teaching that "isotope substitution may not give rise to significant isotope effects in metabolism or clearance." (Id. at 11)
The Patent Owner also submitted evidence establishing that deuteration of three different drugs does not result in a beneficial change in metabolism. In paroxetine8 – another serotonin reuptake inhibitor – deuteration resulted in increased metabolism rather than decreased metabolism. (Id. at 11-12) In phentermine,9 no deuterium effect was observed with an N-di-(tri-deuteromethyl) group – one of the same types of groups found in the claimed analog. (Id. at 12) And tramadol10 (which, like venlafaxine, is metabolized via O- and N-demethylation) "exhibited reduced in vitro metabolism, [and] none of the prepared deuterated versions were superior to tramadol in terms of potency or duration of effect, clearance was not reduced and half-life was not increased." (Id.)
In denying institution of the inter partes review, the Board agreed with the Patent Owner, finding that the Petitioner had not demonstrated that the ordinary artisan would have combined Fogelman with Miwa with a reasonable expectation of arriving at the invention claimed in the '317 patent. (Id. at 13)
The Board was persuaded by the Patent Owner's evidence that a deuteration "strategy will usually not result in significant alterations in overall metabolic clearance of the substrate" and that it "is difficult to predict a priori which effect deuterium may have on a drug's metabolism." (Id. at 14) The Board also found that the Petitioner did not adequately explain why a skilled artisan would seek to prevent metabolism of venlafaxine into ODV, venlafaxine's longer-lived, active metabolite, rather than focus on one of venlafaxine's nonactive metabolites. (Id.) Therefore, the Board concluded that "the ordinary artisan would not have had a reasonable expectation that substitution of all of the nine hydrogens at those sites for deuterium would result in a deuterated [venlafaxine] derivative having enhanced bioavailability and which maintains its activity for a longer period of time." (Id. at 15)
The Board also rejected the Petitioner's allegation that it would have been "obvious to try" to deuterate venlafaxine at the claimed positions, relying upon In re O'Farrell, 853 F.2d 894, 903 (Fed. Cir. 1988). (Id. at 19-20[11]) Here, the Board noted that there are approximately 124,217,727 possible deuterated forms of venlafaxine. And even though Fogelman suggests that metabolism occurs at two main sites, one skilled in the art "would not have had a reasonable expectation that deuteration of those sites would result in enhanced bioavailability" and a maintenance of biological activity for a longer period of time. (Id. at 20-21) The Board noted that the ordinary artisan "would be left with trying the over 100,000,000 possible deuterated forms of [venlafaxine] until possibly arriving at a successful result."
Footnotes
1 "Since deuterium (D) is two-fold more massive than hydrogen (H), it follows that a C-D bond is stronger than the corresponding C-H bond. . . . If a C-H bond is broken during a rate determining step in a chemical reaction . . . then substituting a deuterium for that hydrogen will cause a decrease in the reaction rate, and the process will slow down. This phenomenon is known as the Deuterium Kinetic Isotope Effect (DKIE)." ('317 patent at col. 2:45-58)
2 Fogelman et al. ("Fogelman"), O- and N-demethylation of Venlafaxine In Vitro by Human Liver Microsomes and by Microsomes from cDNA-Transfected Cells: Effect of Metabolic Inhibitors and SSRI Antidepressants, 20 Neuropsychopharmacology 480-490 (1999).
3 Gerald T. Miwa and Anthony Y. H. Lu ("Miwa"), Kinetic Isotope Effects and 'Metabolic Switching' in Cytochrome P450-Catalyzed Reactions, 7 BioEssays 215-19 (1987).
4 The Petitioner also challenged claims 2 and 8 as being obvious over Fogelman, Miwa, and U.S. Application No. 2003/0190351 to Platteeuw and claims 3-7, 9, and 10 as being obvious over Fogelman, Miwa, Platteeuw, and U.S. Patent No. 6,197,828.
5 The Patent Owner did not submit an expert declaration.
6 Citing to Fisher et al., The Complexities Inherent in Attempts to Decrease Drug Clearance by Blocking Sites of CYP-Mediated Metabolism, 9 Current Opinion in Drug Discovery & Development 101-109 (2006).
7 Scott L. Harbeson and Roger D. Tung, Deuterium in Drug Discovery and Development, 46 Annual Reports in Medicinal Chemistry 403-417 (John E. Macor, Ed., 2011).
8 Citing Harbeson and Tung at 413-414.
9 Citing Allan B. Foster, Deuterium Isotope Effects in the Metabolism of Drugs and Xenobiotics: Implications for Drug Design, 14 Advances in Drug Research 1-40 (1985).
10 Citing Shao et al., Derivative of Tramadol for Increased Duration of Effect, 16 Bioorganic & Medicinal Chemistry Letters 691-94 (2006).
11 According to In re O'Farrell, there are two situations in which an "obvious to try" rationale is improper. (Neptune, Paper 25 at 20) The first situation is where one must "vary all parameters or try each of numerous possible choices until one possibly arrived at a successful result where the prior art gave either no indication of which parameters are critical or no direction as to which of many possible choices is likely to be successful." (Id.) The second situation is where one must "explore a new technology or general approach that seemed to be a promising field of experimentation, where the prior art gave only general guidance as to the particular form of the claimed invention or how to achieve it." (Id.)
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.

