In decisions dated April 12, 2016, the USPTO Patent Trial and Appeal Board (PTAB) denied institution as to claims 1-18 of Jazz’s U.S. Patent 8,772,306, finding that neither Ranbaxy Inc. nor Par Pharmaceutical, Inc. had shown a reasonable likelihood of establishing obviousness. Amneal Pharmaceuticals LLC also has filed a petition for IPR of the ‘306 patent. Will the PTAB find its petition more convincing?
The Jazz Zyrem Patent At Issue
As I noted in this article on the April 12, 2016 PTAB decisions, the ‘306 patent is one of listed in the Orange Book for Xyrem®, and has an expiration date of March 15, 2033. Claim 1 recites methods of reducing the dose of gamma-hydroxybutyrate (GBH) when the patient also is being treated with valproate.
1. A method for treating a patient who is suffering from excessive daytime sleepiness, cataplexy, sleep paralysis, apnea, narcolepsy, sleep time disturbances, hypnagogic hallucinations, sleep arousal, insomnia, or nocturnal myoclonus with gamma-hydroxybutyrate (GHB) or a salt thereof, said method comprising: orally administering to the patient in need of treatment at least 5% decrease in an effective dosage amount of the GHB or salt thereof when the patient is receiving a concomitant administration of valproate, an acid, salt, or mixture thereof.
Amneal’s Arguments For Obviousness
Amneal’s petition for IPR cites some of the same pieces of prior art as the Ranbaxy and Par petitions (including the original Xyrem prescribing information) but also cites different references. Notably, the Amneal petition includes a detailed discussion of GHB clearance pathways. This could be significant because one reason the PTAB denied institution of the Ranxbaxy and Par challenges to claims 1-18 was based on Jazz’s evidence that “GHB is eliminated from the body through alternate pathways that are not inhibited by valproate, and these alternate elimination pathways may actually decrease GHB levels, which contributes to the overall unpredictability of combining the two drugs when treating patients for the claimed sleep disorders.”
Amneal’s petition includes this illustration of the “Inhibitory Effect of Valproate on Metabolic Pathways for GHB”:
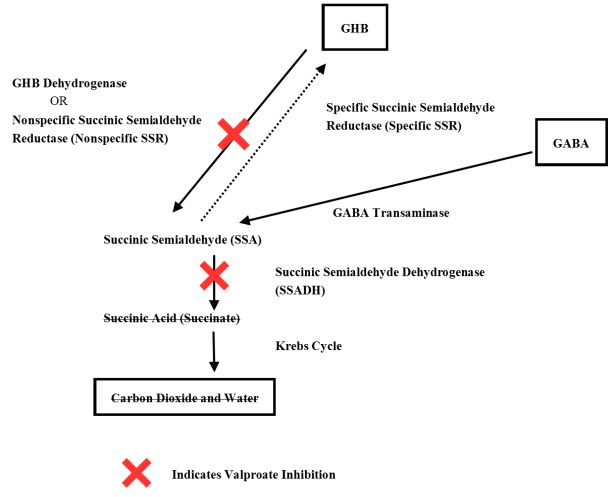
Still, it is not clear that Amneal’s petition addresses all of the deficiencies the PTAB found in the Ranbaxy and Par petitions. For example, Amneal does not appear to have addressed the argument that since valproate was known to interact dangerously, the prior art “teaches away” from the claimed methods by discouraging co-administration altogether. Amneal argues that screening for drug-drug interactions is a “routine practice,” but has it shown that someone of ordinary skill in the art would have had a reasonable expectation that a lower dose of GBH would be suitable?
Jazz has until May 8, 2016 to file a Patent Owner Preliminary Response. Once it does, the PTAB will have three months to decide whether to institute IPR based on Amneal’s arguments.
No Repose For A Patent Owner
The proceedings involving the ‘306 patent illustrate how a patent can be subject to multiple sequential challenges by different petitioners, especially when the patent is asserted against multiple defendants. The Par, Ranbaxy and Amneal IPR petitions may have been filed in response to the ANDA litigation Jazz commenced against those parties. Jazz also has asserted the ‘306 patent against Wockhart Limited (July 2015) and Lupin Ltd. (September 2015). Whether or not Amneal’s IPR is instituted, Wockhart and Lupin might file their own IPR petitions against the ‘306 patent. Indeed, Wockhart is involved in other IPRs of other Xyrem® patents.
The content of this article is intended to provide a general guide to the subject matter. Specialist advice should be sought about your specific circumstances.

